| 086B831 |
0I8Y3P32UF |
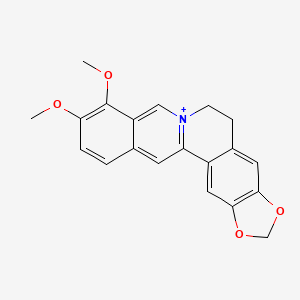
16,17-dimethoxy-5,7-dioxa-13$l^{5}-azapentacyclo[11.8.0.0^{2,10}.0^{4,8}.0^{15,20}]henicosa-1(13),2,4(8),9,14,16,18,20-octaen-13-ylium |
| 2086-83-1 |
3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrido[2,1-a]isoquinolin-5-ylium |
3-[3-(2-ethyl-1,3-thiazol-4-yl)phenyl]-5-{2-[(4-methylphenyl)sulfonyl]ethyl}-1,2,4-oxadiazole |
| 316-41-6 |
34MD1011DM |
5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium chloride |
| 5,6-dihydro-9,10-dimethoxy-benzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium |
7,8,13,13a-tetradehydro-9,10-dimethoxy-2,3-(methylenedioxy)berbinium |
7,8,13,13a-tetradehydro-9,10-dimethoxy-2,3-[methylenebis(oxy)]berbinium |
| 9,10-Dimethoxy-2,3-(methylenedioxy)-7,8,13,13a-tetrahydroberbinium |
9,10-Dimethoxy-5,6-dihydro-7lambda~5~-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinoline |
9,10-Dimethoxy-5,6-dihydro-[1,3]dioxolo-[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium |
| 9,10-Dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinolin-7-ylium |
9,10-Dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinolin-7-ylium |
9,10-Dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinolin-7-ylium chloride |
| 9,10-Dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium |
9,10-dimethoxy-2,3-(methylenedioxy)-7,8,13,13a-tetradehydroberbinium |
9,10-dimethoxy-5,6-dihydro-2H-1,3-dioxoleno[4,5-g]isoquinolino[3,2-a]isoquinol ine |
| 9,10-dimethoxy-5,6-dihydro[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinolin-7-ium |
AB0106411 |
AC-117 |
| ACon1_001957 |
AKOS002141363 |
BBL029198 |
| BCBcMAP01_000112 |
BDBM50203126 |
BERBINIUM, 7,8,13,13a-TETRAHYDRO-9,10-DIMETHOXY-2,3-(METHYLENEDIOXY)- |
| BPBio1_000476 |
BRD-K14796088-003-06-0 |
BRD-K14796088-003-17-7 |
| BRN 3570374 |
BSPBio_000432 |
BSPBio_002156 |
| Benzo(g)-1,3-benzodioxolo(5,6-a)quinolizinium, 5,6-dihydro-9,10-dimethoxy- |
Benzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium, 5,6-dihydro-9,10-dimethoxy- |
Benzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium, 5,6-dihydro-9,10-dimethoxy- (9CI) |
| Benzylpenicillin benzathine, Antibiotic for Culture Media Use Only |
Berbamine sulphate acid |
Berberal |
| Berbericine |
Berberin |
Berberinechloride |
| Berbinium |
Berbinium, 7,8,13,13a-tetradehydro-9,10-dimethoxy-2,3-(methylenedioxy)- |
Berbinium, 7,8,13,13a-tetradehydro-9,10-dimethoxy-2,3-(methylenedioxy)-, chloride |
| C00757 |
C20H18NO4 |
CAS-633-65-8 |
| CCG-35898 |
CHEBI:16118 |
CHEMBL12089 |
| CHEMBL295124 |
CS-0009734 |
CTK6J4626 |
| Coptis rhizome |
DB-050153 |
DB04115 |
| DTXSID9043857 |
DivK1c_000265 |
EINECS 218-229-1 |
| FT-0603587 |
GNF-PF-4545 |
HMS3561D13 |
| HY-N0716 |
IDI1_000265 |
InChI=1/C20H18NO4/c1-22-17-4-3-12-7-16-14-9-19-18(24-11-25-19)8-13(14)5-6-21(16)10-15(12)20(17)23-2/h3-4,7-10H,5-6,11H2,1-2H3/q+ |
| KBio1_000265 |
KBio2_001590 |
KBio2_004158 |
| KBio2_006726 |
KBio3_001656 |
KBioGR_001230 |
| KBioSS_001590 |
LS-43472 |
MCULE-8261808830 |
| MEGxp0_001923 |
Majarine |
N1368 |
| NCGC00016526-01 |
NCGC00016526-02 |
NCGC00016526-03 |
| NCGC00016526-04 |
NCGC00016526-05 |
NCGC00016526-06 |
| NCGC00016526-07 |
NCGC00016526-08 |
NCGC00016526-11 |
| NCGC00091896-03 |
NCI60_001050 |
NCI60_001224 |
| NCI60_004319 |
NCIMech_000354 |
NINDS_000265 |
| NSC646666 |
Prestwick0_000586 |
Prestwick1_000586 |
| Prestwick2_000586 |
Prestwick3_000586 |
Q-200701 |
| Q-200702 |
Q176525 |
SBI-0051613.P002 |
| SCHEMBL25632 |
SDCCGMLS-0066718.P001 |
SMP1_000298 |
| SPBio_000708 |
SPBio_002651 |
SR-01000711827-5 |
| ST055798 |
STK870320 |
Spectrum2_000894 |
| Spectrum3_000618 |
Spectrum4_000785 |
Spectrum5_001458 |
| Spectrum_001110 |
Thalsine |
UNII-0I8Y3P32UF |
| UPCMLD-DP032 |
UPCMLD-DP032:001 |
Umbellatin |
| Umbellatine |
Umbellatine (6CI) |
W-2449 |
| YBHILYKTIRIUTE-UHFFFAOYSA-N |
ZINC3779067 |
berberine |
| berberine dimer |
chloride |
cid_12456 |
| inverted exclamation markY97% |