D0259 | Ciprofloxacin
S
J
S03AA07 Ciprofloxacin
[S03AA] Antiinfectives
[S03A] ANTIINFECTIVES
[S03] OPHTHALMOLOGICAL AND OTOLOGICAL PREPARATIONS
[S] Sensory organs
S02AA15 Ciprofloxacin
[S02AA] Antiinfectives
[S02A] ANTIINFECTIVES
[S02] OTOLOGICALS
[S] Sensory organs
S01AE03 Ciprofloxacin
[S01AE] Fluoroquinolones
[S01A] ANTIINFECTIVES
[S01] OPHTHALMOLOGICALS
[S] Sensory organs
J01RA12 Ciprofloxacin and ornidazole
[J01RA] Combinations of antibacterials
[J01R] COMBINATIONS OF ANTIBACTERIALS
[J01] ANTIBACTERIALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J01RA11 Ciprofloxacin and tinidazole
[J01RA] Combinations of antibacterials
[J01R] COMBINATIONS OF ANTIBACTERIALS
[J01] ANTIBACTERIALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J01RA10 Ciprofloxacin and metronidazole
[J01RA] Combinations of antibacterials
[J01R] COMBINATIONS OF ANTIBACTERIALS
[J01] ANTIBACTERIALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J01MA02 Ciprofloxacin
[J01MA] Fluoroquinolones
[J01M] QUINOLONE ANTIBACTERIALS
[J01] ANTIBACTERIALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | > 400 µM | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | decrease | EC20 | 36 |
| RESPIRATION | 195.0 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | increase | EC20 | 36 |
| RESPIRATION | ND | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | Negative | EC20 | 36 |
| RESPIRATION | decrease | 307 | ||||||
| ELECTRON TRANSPORT CHAIN | decrease | 307 | ||||||
| GENERATION OF LACTATE | Increase | 307 | ||||||
| SWELLING | > 400 µM | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | increase | EC20 | 36 |
| ROS PRODUCTION | Increase | 307 | ||||||
| MITOCHONDRIAL DNA METABOLIC PROCESS | decrease | 307 | ||||||
| MITOCHONDRIAL DNA METABOLIC PROCESS | decrease | 307 | ||||||
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | Inhibition | 307 | ||||||
| Succinate dehydrogenase | ND | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | Negative | EC20 | 36 |
| Succinate dehydrogenase | Inhibition | 307 | ||||||
| Cytochrome c oxidase | Inhibition | 307 | ||||||
| Reactive oxygen species | increase | 307 | ||||||
| Cytochrome c | > 400 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Warning |
Aggregated GHS information provided by 97 companies from 10 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 56 of 97 companies. For more detailed information, please visit ECHA C&L website Of the 9 notification(s) provided by 41 of 97 companies with hazard statement code(s): H319 (14.63%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H361 (21.95%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H412 (63.41%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P264, P273, P280, P281, P305+P351+P338, P308+P313, P337+P313, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | subcutaneous | > 1gm/kg (1000mg/kg) | Zhongguo Yaoxue Zazhi. Chinese Pharmacuetical Journal. Vol. 27, Pg. 297, 1992. | |
| mouse | LD50 | intraperitoneal | 1165mg/kg (1165mg/kg) | Ensho. Japanese Journal of Inflammation. Vol. 11, Pg. 343, 1991. | |
| mouse | LD50 | oral | 5gm/kg (5000mg/kg) | Journal of Medicinal Chemistry. Vol. 33, Pg. 1344, 1990. | |
| women | TDLo | oral | 40mg/kg/2D-I (40mg/kg) | American Journal of Medicine. Vol. 87, Pg. 589, 1989. | |
| mouse | LD50 | intravenous | 122mg/kg (122mg/kg) | Korean Journal of Toxicology. Vol. 8, Pg. 161, 1993. | |
| rat | LD50 | oral | > 2gm/kg (2000mg/kg) | Zhongguo Yaoxue Zazhi. Chinese Pharmacuetical Journal. Vol. 27, Pg. 297, 1992. | |
| rat | LD50 | intravenous | 207mg/kg (207mg/kg) | Korean Journal of Toxicology. Vol. 8, Pg. 161, 1993. | |
| man | TDLo | oral | 21mg/kg/3D-I (21mg/kg) | Annals of Pharmacotherpy. Vol. 29, Pg. 84, 1995. | |
| women | TDLo | oral | 10mg/kg (10mg/kg) | Allergy. Vol. 50(Suppl, | |
| women | TDLo | multiple routes | 40mg/kg/6D-I (40mg/kg) | Acta Clinica Belgica. Vol. 49, Pg. 173, 1994. | |
| man | TDLo | oral | 21429ug/kg/3D (21.429mg/kg) | Annals of Pharmacotherpy. Vol. 26, Pg. 930, 1992. | |
| women | TDLo | oral | 135mg/kg/3D-I (135mg/kg) | kidney, ureter, and bladder: "changes in tubules (including acute renal failure, acute tubular necrosis)" | Archives of Internal Medicine. Vol. 153, Pg. 1258, 1993. |
| man | TDLo | oral | 200mg/kg (200mg/kg) | Annals of Pharmacotherpy. Vol. 28, Pg. 805, 1994. | |
| man | LDLo | oral | 5714ug/kg/2D- (5.714mg/kg) | Lancet. Vol. 343, Pg. 738, 1994. | |
| man | TDLo | oral | 129mg/kg/6D-I (129mg/kg) | behavioral: regidity | Journal of Clinical Psychiatry. Vol. 54, Pg. 115, 1993. |
| women | TDLo | oral | 280mg/kg/2W-I (280mg/kg) | American Journal of Kidney Diseases. Vol. 22, Pg. 598, 1993. | |
| mouse | LD50 | subcutaneous | > 1gm/kg (1000mg/kg) | Zhongguo Yaoxue Zazhi. Chinese Pharmacuetical Journal. Vol. 27, Pg. 297, 1992. | |
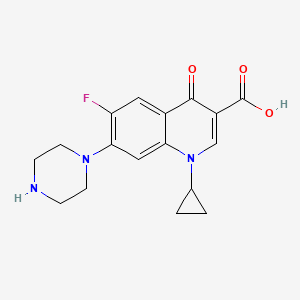
| 1-CYCLOPROPYL-6-FLUORO-4-OXO-7-PIPERAZIN-1-YL-1,4-DIHYDROQUINOLINE-3-CARBOXYLIC ACID | 1-Cyclopropyl-6-fluoro-1,4-dihydro 4-oxo-7-[1-piperazinyl)-quinoline-3-carboxylic Acid | 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7- (1-piperazinyl)-3-quinoline-carboxylic acid |
| 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3- quinolinecarboxylic acid | 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline-carboxylic acid | 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid |
| 1-Cyclopropyl-6-fluoro-1,4-dihydro-7-(1-piperazinyl)-4-oxo-3-quinoline carboxylic acid | 1-Cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydro-3-quinolinecarboxylic acid | 1-Cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydro-quinoline-3-carboxylic acid |
| 1-Cyclopropyl-6-fluoro-7-(4-methyl-piperazin-1-yl)-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid | 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-quinoline -3-carboxylic acid | 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-quinoline-3-carboxylic acid |
| 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)quinoline-3-carboxylic acid | 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1piperazinyl)-3quinolinecarboxylic acid | 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(piperazin-1-yl)-quinoline-3-carboxylic acid |
| 1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid | 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydroquinoline-3-carboxylic acid hydrochloride | 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-yl-quinoline-3-carboxylic acid |
| 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-ylquinoline-3-carboxylic acid | 1-cyclopropyl-6-fluoro-4-oxo-7-piperazinylhydroquinoline-3-carboxylic acid | 1-cyclopropyl-6-fluoro-7-(piperazin-1-yl)-1,4-dihydro-4-oxoquinoline-3-carboxylic acid |
| 1-cyclopropyl-6-fluoro-7-hexahydro-1-pyrazinyl-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid | 3-Quinolinecarboxylic acid, 1,4-dihydro-1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)- | 3-Quinolinecarboxylic acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)- |
| 3-Quinolinecarboxylic acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-, hydrochloride | 3-Quinolinecarboxylic acid,1,4-dihydro-1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl) | 3-Quinolinecarboxylicacid, 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)- |
| 5E8K9I0O4U | 721C331 | 85721-33-1 |
| A-8526 | AB0011928 | AB1009412 |
| AC-7613 | AK163192 | AKOS000269653 |
| ALBB-015909 | ANHYDROUS CIPROFLOXACIN | ARONIS020379 |
| Alcon Cilox | AuriPro | BAY q 3939 |
| BAY-Q-3939 | BAY-o 9867 | BAYQ3939 |
| BBL005612 | BCP28586 | BDBM21690 |
| BIDD:GT0205 | BPBio1_000140 | BRD-K04804440-311-02-3 |
| BRN 3568352 | BSPBio_000126 | BSPBio_003344 |
| Bacquinor | Baflox | Bay 09867 |
| Bay o 9867 | Bay o 9867 (*Hydrochloride*) | Bay-09867 |
| Bernoflox | Bi-Cipro | C05349 |
| C17H18FN3O3 | CAS-93107-08-5 | CBMicro_048498 |
| CCG-39345 | CCRIS 5241 | CHEBI:100241 |
| CHEMBL8 | CIPRO IN DEXTROSE 5% IN PLASTIC CONTAINER | CIPRO IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER |
| CIPROFLOXACIN EXTENDED RELEASE | CPD000471901 | CPFX |
| CS-2410 | CTK3E8144 | Certified Reference Material |
| Cetraxal | Ciflox | Cifloxin |
| Cilab | Ciloxan (*Hydrochloride*) | Ciplus |
| Ciprecu | Ciprine | Ciprinol |
| Cipro | Cipro (*Hydrochloride*) | Cipro (TN) |
| Cipro IV | Cipro XR | Ciprobay |
| Ciprobay Uro | Ciprocinol | Ciprodar |
| Ciproflox | Ciprofloxacin (Cipro) | Ciprofloxacin (JP17/USP/INN) |
| Ciprofloxacin Hydrchloride | Ciprofloxacin [USAN:INN:BAN] | Ciprofloxacin [USAN:USP:INN:BAN] |
| Ciprofloxacin monohydrochloride | Ciprofloxacin, 98% | Ciprofloxacin, >=98.0% (HPLC) |
| Ciprofloxacin, Antibiotic for Culture Media Use Only | Ciprofloxacin, European Pharmacopoeia (EP) Reference Standard | Ciprofloxacin, Pharmaceutical Secondary Standard |
| Ciprofloxacin, United States Pharmacopeia (USP) Reference Standard | Ciprofloxacin, VETRANAL(TM), analytical standard | Ciprofloxacin,(S) |
| Ciprofloxacina | Ciprofloxacine | Ciprofloxacine [INN-French] |
| Ciprofloxacino | Ciprofloxacino [INN-Spanish] | Ciprofloxacinum |
| Ciprofloxacinum [INN-Latin] | Ciprogis | Ciprolin |
| Ciprolon | Cipromycin | Ciproquinol |
| Ciprowin | Ciproxan | Ciproxin |
| Ciproxina | Ciproxine | Ciriax |
| Citopcin | Cixan | Corsacin |
| Cycin | Cyprobay | D00186 |
| DB00537 | DTXSID8022824 | DivK1c_000095 |
| Eni | FT-0601635 | Fimoflox |
| HMS1922E18 | HMS2090O07 | HMS2093I03 |
| HMS500E17 | HSDB 6987 | HY-B0356 |
| IDI1_000095 | Ipiflox | Italnik |
| J10137 | KBio1_000095 | KBio2_000642 |
| KBio2_003210 | KBio2_005778 | KBio3_002846 |
| KBioGR_001567 | KBioSS_000642 | KS-000002BQ |
| KS-00004C43 | KS-5006 | LS-141563 |
| Linhaliq | Linhaliq [Liposomal Formulation] | Loxan |
| MCULE-8631780654 | MFCD00185755 | MLS001336035 |
| MLS006011837 | MYSWGUAQZAJSOK-UHFFFAOYSA-N | NCGC00016959-01 |
| NCGC00016959-02 | NCGC00016959-03 | NCGC00016959-04 |
| NCGC00016959-05 | NCGC00016959-07 | NCGC00095058-01 |
| NCGC00095058-02 | NCGC00178128-01 | NINDS_000095 |
| NSC-758467 | NSC620634 | NSC758467 |
| Oprea1_008239 | Oprea1_313572 | Otiprio |
| Pharmakon1600-01503614 | Prestwick0_000113 | Prestwick1_000113 |
| Prestwick2_000113 | Prestwick3_000113 | Probiox |
| Proflaxin | Proflox | Proksi 250 |
| Proksi 500 | Q256602 | Quinolid |
| Quintor | RKL10073 | Rancif |
| Roxytal | SAM002264604 | SBB012554 |
| SBI-0048462.P003 | SC-17889 | SCHEMBL2900 |
| SMP1_000125 | SMR000471901 | SPBio_001474 |
| SPBio_002065 | SPECTRUM1503614 | SR-05000001863 |
| SR-05000001863-1 | SR-05000001863-3 | ST024751 |
| ST24046329 | STK021082 | Septicide |
| Sophixin Ofteno | Spectrum2_001567 | Spectrum3_001872 |
| Spectrum4_000874 | Spectrum5_001089 | Spectrum_000162 |
| Spitacin | Superocin | TX-017484 |
| UNII-5E8K9I0O4U | Unex | Velmonit |
| Z56933707 | ZINC20220 | Zumaflox |
| ciprofloxacin | ciprofloxacin-cipro | rubrum |
| s2027 |
| DrugBank Name | Ciprofloxacin |
| DrugBank | DB00537 |
| CAS Number | 85721-33-1, 86393-32-0 |
| PubChem Compound | 2764 |
| KEGG Compound ID | C05349 |
| KEGG Drug | D00186 |
| PubChem.Substance | 46504733 |
| ChEBI | 100241 |
| PharmGKB | PA449009 |
| ChemSpider | 2662 |
| BindingDB | 21690.0 |
| TTD | DAP001360 |
| Wikipedia | Ciprofloxacin |
| HET | CPF |
| DPD | 93|8327 |