D0432 | tetracyclines
S
J
A
D
S03AA02 Tetracycline
[S03AA] Antiinfectives
[S03A] ANTIINFECTIVES
[S03] OPHTHALMOLOGICAL AND OTOLOGICAL PREPARATIONS
[S] Sensory organs
S02AA08 Tetracycline
[S02AA] Antiinfectives
[S02A] ANTIINFECTIVES
[S02] OTOLOGICALS
[S] Sensory organs
S01AA09 Tetracycline
[S01AA] Antibiotics
[S01A] ANTIINFECTIVES
[S01] OPHTHALMOLOGICALS
[S] Sensory organs
J01RA08 Tetracycline and oleandomycin
[J01RA] Combinations of antibacterials
[J01R] COMBINATIONS OF ANTIBACTERIALS
[J01] ANTIBACTERIALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J01AA20 Combinations of tetracyclines
[J01AA] Tetracyclines
[J01A] TETRACYCLINES
[J01] ANTIBACTERIALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J01AA07 Tetracycline
[J01AA] Tetracyclines
[J01A] TETRACYCLINES
[J01] ANTIBACTERIALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
D06AA04 Tetracycline
[D06AA] Tetracycline and derivatives
[D06A] ANTIBIOTICS FOR TOPICAL USE
[D06] ANTIBIOTICS AND CHEMOTHERAPEUTICS FOR DERMATOLOGICAL USE
[D] Dermatological drugs
A02BD08 Bismuth subcitrate, tetracycline and metronidazole
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A02BD02 Lansoprazole, tetracycline and metronidazole
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A01AB13 Tetracycline
[A01AB] Antiinfectives and antiseptics for local oral treatment
[A01A] STOMATOLOGICAL PREPARATIONS
[A01] STOMATOLOGICAL PREPARATIONS
[A] Alimentary tract and metabolism
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| PROTONOPHORIC UNCOUPLING | 278 | |||||||
| MEMBRANE POTENTIAL | decrease | 307 | ||||||
| ELECTRON TRANSPORT CHAIN | decrease | 307 | ||||||
| TCA | mouse | liver mitochondria | Tricarboxylic acid cycle activity was assessed by the in vitro formation of [14C]CO2 from [1-14C]acetylcoenzyme A by mouse liver mitochondria. | affect | 236 | |||
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | mouse | liver mitochondria | affect | 236 | ||||
| MITOCHONDRIAL PROTEIN TRANSLATION | 2.1 ± 0.5 μM | rat | liver mitochondria | Mitochondrial protein synthesis assay (The incorporation of [35S]methionine into mitochondrial protein was determined by a filter paper disk assay ) | IC50 | 281 | ||
| MITOCHONDRIAL PROTEIN TRANSLATION | decrease | 307 | ||||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 49 companies from 2 notifications to the ECHA C&L Inventory. H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P264, P270, P301+P312, P330, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | subcutaneous | > 5gm/kg (5000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 27, Pg. 195, 1996. | |
| mouse | LD50 | subcutaneous | > 4gm/kg (4000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 27, Pg. 195, 1996. | |
| mouse | LD50 | oral | > 5gm/kg (5000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 27, Pg. 195, 1996. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 27, Pg. 195, 1996. | |
| mouse | LD50 | intravenous | 1638mg/kg (1638mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 27, Pg. 195, 1996. | |
| rat | LD50 | intravenous | 1310mg/kg (1310mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 27, Pg. 195, 1996. | |
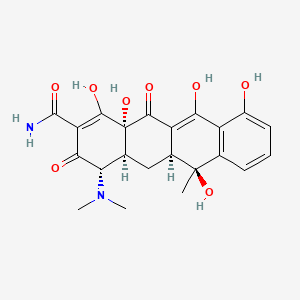
| (4S,4aS,5aS,12aS)-4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide | (4S,4aS,5aS,6S,12aS)-2-[Amino(hydroxy)methylene]-4beta-(dimethylamino)-1,2,3,4,4a,5,5a,6,11,12a-decahydro-6alpha,10,12,12abeta-tetrahydroxy-6-methylnaphthacene-1,3,11-trione | (4S,4aS,5aS,6S,12aS)-4-(Dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-2-tetracenecarboxamide |
| (4S,4aS,5aS,6S,12aS)-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide | (4S,4aS,5aS,6S,12aS)-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide | (4S,6S,12aS,4aS,5aS)-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,1 1-dioxo-4,5,6,12a,4a,5a-hexahydronaphthacene-2-carboxamide; |
| 1369-EP2269978A2 | 1369-EP2269985A2 | 1369-EP2269991A2 |
| 1369-EP2270008A1 | 1369-EP2275420A1 | 1369-EP2284150A2 |
| 1369-EP2284151A2 | 1369-EP2284152A2 | 1369-EP2284153A2 |
| 1369-EP2284155A2 | 1369-EP2284156A2 | 1369-EP2284164A2 |
| 1369-EP2287140A2 | 1369-EP2287148A2 | 1369-EP2287150A2 |
| 1369-EP2289871A1 | 1369-EP2289891A2 | 1369-EP2292088A1 |
| 1369-EP2292590A2 | 1369-EP2292612A2 | 1369-EP2292617A1 |
| 1369-EP2295402A2 | 1369-EP2295419A2 | 1369-EP2298732A1 |
| 1369-EP2301534A1 | 1369-EP2301912A2 | 1369-EP2301913A1 |
| 1369-EP2301914A1 | 1369-EP2301916A2 | 1369-EP2305637A2 |
| 1369-EP2305640A2 | 1369-EP2305662A1 | 1369-EP2307027A1 |
| 1369-EP2308832A1 | 1369-EP2308863A1 | 1369-EP2308883A1 |
| 1369-EP2311451A1 | 1369-EP2311796A1 | 1369-EP2311797A1 |
| 1369-EP2311798A1 | 1369-EP2311799A1 | 1369-EP2311822A1 |
| 1369-EP2314583A1 | 1369-EP2316450A1 | 1369-EP2371797A1 |
| 1369-EP2371798A1 | 1369-EP2371800A1 | 1369-EP2371804A1 |
| 2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo- | 2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, (4S,4aS,5aS,6S,12aS)- | 2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, (4S-(4alpha,4aalpha,5aalpha,6beta,12aalpha))- |
| 4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,1 0,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide | 6-Methyl-1,11-dioxy-2-naphthacenecarboxamide | 60-54-8 |
| 6416-04-2 (3H2O) | AB00053550-04 | AB00053550_05 |
| AB00053550_06 | AKOS024277860 | AKOS026749977 |
| Abramycin | Abricycline | Achromycin |
| Achromycin (naphthacene derivative) | Achromycin V | Agromicina |
| Ambramicina | Ambramycin | Amycin |
| BDBM50237605 | BPBio1_000242 | BSPBio_000220 |
| BSPBio_001950 | Bio-tetra | Biocycline |
| C06570 | CAS-60-54-8 | CCRIS 9483 |
| CHEBI:27902 | CPD003601804 | CS-8188 |
| Cefracycline | Cefracycline suspension | Centet (base) |
| Ciclibion | Copharlan | Criseociclina |
| Cyclomycin | D00201 | DB00759 |
| DSSTox_CID_3645 | DSSTox_GSID_23645 | DSSTox_RID_77127 |
| DTXSID7023645 | Democracin | Deschlorobiomycin |
| DivK1c_000827 | E701 | EC 200-481-9 |
| EINECS 200-481-9 | Economycin | F8VB5M810T |
| FT-0696567 | HMS2090B04 | HSDB 3188 |
| HY-A0107 | Hostacyclin | IDI1_000827 |
| KBio1_000827 | KBio2_001514 | KBio2_004082 |
| KBio2_006650 | KBio3_001450 | KBioGR_000783 |
| KBioSS_001514 | Lexacycline | Limecycline |
| Liquamycin (Veterinary) | MCULE-3752961261 | MFCD00151232 |
| Mericycline | Micycline | NCGC00017323-03 |
| NCGC00017323-04 | NCGC00017323-05 | NCGC00017323-07 |
| NCGC00142507-02 | NCGC00254063-01 | NINDS_000827 |
| NSC-108579 | NSC108579 | NWXMGUDVXFXRIG-WESIUVDSSA-N |
| Neocycline | OFVLGDICTFRJMM-WESIUVDSSA-N | Omegamycin |
| Orlycycline | Piracaps (base) | Polycycline |
| Polycycline (VAN) | Polycycline (antibiotic) | Polyotic |
| Prestwick0_000140 | Prestwick1_000140 | Prestwick2_000140 |
| Prestwick3_000140 | Purocyclina | Q193045 |
| RKL10088 | Roviciclina | SAM002554934 |
| SBI-0051530.P003 | SC-81153 | SCHEMBL2116649 |
| SCHEMBL2116661 | SCHEMBL21271987 | SCHEMBL3098 |
| SCHEMBL537050 | SK-Tetracycline | SPBio_001457 |
| SPBio_002159 | SR-01000000212 | SR-01000000212-3 |
| ST019403 | Solvocin | Spectrum2_001329 |
| Spectrum3_000565 | Spectrum4_000352 | Spectrum5_001112 |
| Spectrum_001034 | Sumycin (TN) | Sumycin syrup |
| Supramycin | Sustamycin | T-125 |
| Tetra-Co | Tetrabid Organon | Tetrabon |
| Tetraciclina | Tetraciclina [INN-Spanish] | Tetracycl |
| Tetracyclin | Tetracycline (JAN/USP/INN) | Tetracycline (internal use) |
| Tetracycline I | Tetracycline II | Tetracycline [USP:INN:BAN:JAN] |
| Tetracycline base | Tetracycline, >=88.0% (HPLC) | Tetracycline, >=98.0% (NT) |
| Tetracycline, EP grade | Tetracyclinehydrate | Tetracyclinum |
| Tetracyclinum [INN-Latin] | Tetracyn | Tetradecin |
| Tetrafil | Tetraverine | Tetrazyklin |
| Tetrex | Tox21_300150 | Tsiklomistsin |
| Tsiklomitsin | UNII-F8VB5M810T | Veracin |
| Vetacyclinum | Vetquamycin | Vetquamycin-324 (free base) |
| Z2144222809 | ZINC100303069 | ZINC102229720 |
| ZINC84441937 | tetracycline |
| DrugBank Name | Tetracycline |
| DrugBank | DB00759 |
| CAS Number | 115074-43-6, 60-54-8, 64-75-5 |
| PubChem Compound | 54675776 |
| KEGG Drug | D00201 |
| ChEBI | 27902 |
| PharmGKB | PA451640 |
| ChemSpider | 10257122 |
| BindingDB | 50237605.0 |
| TTD | DAP001527 |
| Wikipedia | Tetracycline |