D1379 | atorvastatin
C
C10BX15 Atorvastatin and perindopril
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BX12 Atorvastatin, acetylsalicylic acid and perindopril
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BX11 Atorvastatin, amlodipine and perindopril
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BX08 Atorvastatin and acetylsalicylic acid
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BX06 Atorvastatin, acetylsalicylic acid and ramipril
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BX03 Atorvastatin and amlodipine
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BA05 Atorvastatin and ezetimibe
[C10BA] HMG CoA reductase inhibitors in combination with other lipid modifying agents
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10AA05 Atorvastatin
[C10AA] HMG CoA reductase inhibitors
[C10A] LIPID MODIFYING AGENTS, PLAIN
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| OPENING OF PERMEABILITY TRANSITION PORE (PTP) | 50 µM | 1 hour | Human | HepG2 | High-content screening assay | Decrease | MEC | 306 |
| MEMBRANE POTENTIAL | 100 µM | 1 hour | Human | HepG2 | High-content screening assay | Increase | MEC | 306 |
| ELECTRON TRANSPORT CHAIN | C2C12 myoblasts | affect | 180 | |||||
| ROS PRODUCTION | 100 µM | 1 hour | Human | HepG2 | High-content screening assay | Increase | MEC | 306 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| Qo site (Qp site or ubiquinol oxidation site) | C2C12 myoblasts | inhibitor | 180 | |||||
| Reactive oxygen species | 100 µM | 1 hour | Human | HepG2 | High-content screening assay | increase | MEC | 306 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Warning |
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory. H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] H361 (100%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H362 (100%): May cause harm to breast-fed children [Reproductive toxicity, effects on or via lactation] |
P201, P202, P260, P261, P263, P264, P270, P271, P280, P281, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
  |
Warning |
Aggregated GHS information provided by 79 companies from 14 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 3 of 79 companies. For more detailed information, please visit ECHA C&L website Of the 13 notification(s) provided by 76 of 79 companies with hazard statement code(s): H373 (48.68%): Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H402 (38.16%): Harmful to aquatic life [Hazardous to the aquatic environment, acute hazard] H411 (46.05%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] H412 (40.79%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P260, P273, P314, P391, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
 |
Warning |
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory. H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] |
P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
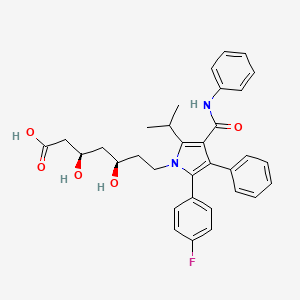
| (3R,5R)-7-(2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)-3,5-dihydroxy | (3R,5R)-7-(2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoic acid | (3R,5R)-7-[2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-phenylcarbamoylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid |
| (3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid | (3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid | (3R,5R)-7-[2-(4-fluorophenyl)-5-(1-methylethyl)-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid |
| (3R,5R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]-3,5-dihydroxy-heptanoic acid | (3R,5R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-phenylcarbamoyl-pyrrol-1-yl]-3,5-dihydroxy-heptanoic acid | (3R,5R)-7-[3-(anilinocarbonyl)-5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid |
| (3R,5R)-7-[3-(anilinocarbonyl)-5-(4-fluorophenyl)-4-phenyl-2-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid | (R-(R*,R*))-2-(4-Fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-((phenylamino)carbonyl)-1H-pyrrole-1-heptanoic acid | (betaR,deltaR)-2-(p-Fluorophenyl)-beta,delta-dihydroxy-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrole-1-heptanoic acid |
| 110862-48-1 | 134523-00-5 | 134523-03-8 |
| 1H-Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-((phenylamino)carbonyl)-, (R-(R*,R*))- | 1H-Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-((phenylamino)carbonyl)-, (betaR,deltaR)- | 28052-EP2269989A1 |
| 28052-EP2269990A1 | 28052-EP2270011A1 | 28052-EP2270505A1 |
| 28052-EP2272825A2 | 28052-EP2272841A1 | 28052-EP2280001A1 |
| 28052-EP2284158A1 | 28052-EP2287165A2 | 28052-EP2287166A2 |
| 28052-EP2292600A1 | 28052-EP2292620A2 | 28052-EP2295406A1 |
| 28052-EP2295409A1 | 28052-EP2295417A1 | 28052-EP2295422A2 |
| 28052-EP2298731A1 | 28052-EP2298742A1 | 28052-EP2298745A1 |
| 28052-EP2298769A1 | 28052-EP2298772A1 | 28052-EP2298776A1 |
| 28052-EP2298779A1 | 28052-EP2301923A1 | 28052-EP2301931A1 |
| 28052-EP2301936A1 | 28052-EP2308839A1 | 28052-EP2308878A2 |
| 28052-EP2314588A1 | 523A005 | 7-[2-(4-FLUORO-PHENYL)-5-ISOPROPYL-3-PHENYL-4-PHENYLCARBAMOYL-PYRROL-1-YL]- 3,5-DIHYDROXY-HEPTANOIC ACID |
| 7-[2-(4-FLUORO-PHENYL)-5-ISOPROPYL-3-PHENYL-4-PHENYLCARBAMOYL-PYRROL-1-YL]-3,5-DIHYDROXY-HEPTANOIC ACID | A0JWA85V8F | AC-9386 |
| ACT03225 | AKOS000281127 | AS-35260 |
| ATORVASTATIN CALCIUM | ATORVASTATIN;Atorvastatin calcium;Atorvastatin Calcium | Atofast |
| Ator | Atorcor | Atorin |
| Atorlip | Atorvastatin & Primycin | Atorvastatin (INN) |
| Atorvastatin (Relative Stereo) | Atorvastatin [INN:BAN] | Atorvastatin calcium salt |
| BDBM22164 | BIDD:GT0336 | BR-58267 |
| BRD-K69726342-001-02-6 | C06834 | C33H35FN2O5 |
| CAS-134523-00-5 | CCG-221172 | CCRIS 7159 |
| CHEBI:39548 | CHEMBL1487 | CI 981 |
| CS-2798 | CTK4B9231 | Cardyl |
| Cardyl | D07474 | DB01076 |
| DSSTox_CID_9868 | DSSTox_GSID_29868 | DSSTox_RID_78825 |
| DTXSID60274003 | DTXSID8029868 | GTPL2949 |
| H942 | HMS3715L05 | HSDB 7039 |
| HY-B0589 | LS-136975 | Lipilou |
| Lipilou | Lipinon | Lipitor |
| Lipitor (TN) | Lipitor(TM) | MCULE-2368532812 |
| MRF-0000761 | NCGC00159458-02 | NCGC00159458-03 |
| NCGC00159458-20 | NCGC00255181-01 | Q668093 |
| S-2492 | SCHEMBL3831 | SR-01000872702 |
| SR-01000872702-1 | Sortis (TN) | Sotis |
| Torvast | Torvast | Tox21_302417 |
| Tozalip | Tozalip | UNII-36TN91XZ0V component XUKUURHRXDUEBC-KAYWLYCHSA-N |
| UNII-A0JWA85V8F | Xavator | Y-8867 |
| ZINC3920719 | atorvastatin | atorvastatina |
| atorvastatine | atorvastatinum | atrovastin |
| rel-Atorvastatin | s5715 |