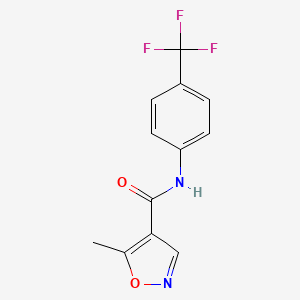
Drug
D1423 | Leflunomide
L
L04AA13 Leflunomide
[L04AA] Selective immunosuppressants
[L04A] IMMUNOSUPPRESSANTS
[L04] IMMUNOSUPPRESSANTS
[L] Antineoplastic and immunomodulating agents
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| Quinol--cytochrome-c reductase | 50 μM | 48 hr | MEF cells | Mitochondrial Complex III Activity Assay kit(BioVision) | inhibit | 295 | ||
| Dihydroorotate dehydrogenase | 50 μM | 48 hr | HeLa cells, C2C12 muscle cells, MEF cells | Mfn2 and Mfn1 mRNA levels; MFN2, MFN1, and porin protein levels; images of mitochondrial morphology ; | inhibit | 295 | ||