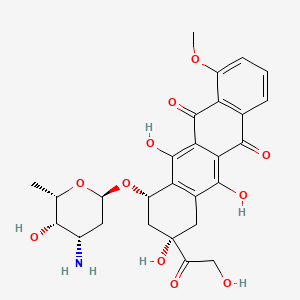
D0043 | Doxorubicin
L
L01DB01 Doxorubicin
[L01DB] Anthracyclines and related substances
[L01D] CYTOTOXIC ANTIBIOTICS AND RELATED SUBSTANCES
[L01] ANTINEOPLASTIC AGENTS
[L] Antineoplastic and immunomodulating agents
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 8.9 µM | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | decrease | EC20 | 36 |
| REDOX CYCLING | 278 | |||||||
| RESPIRATION | 15.9 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | decrease | EC20 | 36 |
| RESPIRATION | ND | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | Negative | EC20 | 36 |
| ELECTRON TRANSPORT CHAIN | decrease | 36 | ||||||
| GLUCOSE GALACTOSE IC50 RATIO | 0.5 | LUHMES (Lund human mesencephalic) cells | Glc–Gal–NeuriTox assay | Negative | EC25(NA) [Glc/Gal] | 326 | ||
| IRON HOMEOSTASIS | 197 | |||||||
| SWELLING | 85 µM | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | increase | EC20 | 36 |
| MITOCHONDRIAL DYNAMICS | 7.2 μM DXR for 24 hrs | Human | fibroblasts | 247 | ||||
| OXIDATIVE STRESS | 250 | |||||||
| ROS PRODUCTION | increase | 197 | ||||||
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 15.9 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | inhibit | EC20 | 36 |
| Succinate dehydrogenase | ND | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | Negative | EC20 | 36 |
| Glycerol-3-phosphate dehydrogenase, mitochondrial | inhibitor | 162 | ||||||
| Cytochrome c | 23.3 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 38 companies from 1 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H350 (100%): May cause cancer [Danger Carcinogenicity] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P264, P270, P281, P301+P312, P308+P313, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intraperitoneal | 16mg/kg (16mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 6, Pg. 1075, 1972. | |
| man | LDLo | unreported | 243ug/kg (0.243mg/kg) | Postgraduate Medical Journal. Vol. 68, Pg. 69, 1992. | |
| mouse | LD50 | intraperitoneal | 10700ug/kg (10.7mg/kg) | Huaxi Yike Daxue Xuebao. Journal of West China University of Medical Sciences. Vol. 20, Pg. 303, 1989. | |
| human | LDLo | intravenous | 400ug/kg (0.4mg/kg) | Cancer Chemotherapy Reports, Part 3. Vol. 3, Pg. 33, 1972. | |
| hamster | LD10 | parenteral | 3500ug/kg (3.5mg/kg) | Journal of Surgical Oncology. Vol. 15, Pg. 355, 1980. | |
| mouse | LD50 | unreported | 21900ug/kg (21.9mg/kg) | Biochemical Pharmacology. Vol. 38, Pg. 167, 1989. | |
| mouse | LD50 | intravenous | 10mg/kg (10mg/kg) | Journal of Antibiotics. Vol. 45, Pg. 1373, 1992. | |
| human | TDLo | intravenous | 380mg/kg/31W (380mg/kg) | Cancer Vol. 34, Pg. 518, 1974. | |
| rabbit | LDLo | intrapleural | 400ug/kg (0.4mg/kg) | Pharmacology and Toxicology Vol. 62, Pg. 84, 1988. | |
| rat | LD50 | unreported | 7mg/kg (7mg/kg) | Antibiotiki i Khimioterapiya. Antibiotics and Chemotherapy. Vol. 34, Pg. 216, 1989. | |
| rat | LD50 | intravenous | 10510ug/kg (10.51mg/kg) | Toxicology and Applied Pharmacology. Vol. 79, Pg. 412, 1985. | |
| mammal (species unspecified) | LD50 | intraperitoneal | 8500ug/kg (8.5mg/kg) | Antibiotiki. Vol. 29, Pg. 748, 1984. | |
| rabbit | LD50 | intravenous | 5mg/kg (5mg/kg) | Yiyao Gongye. Pharmaceutical Industry. Vol. 17, Pg. 72, 1986. | |
| human | TDLo | intravenous | 15mg/kg/D (15mg/kg) | Cancer Vol. 34, Pg. 518, 1974. | |
| mouse | LD50 | oral | 570mg/kg (570mg/kg) | Antibiotiki. Vol. 28, Pg. 298, 1983. | |
| dog | LD50 | intravenous | 2400ug/kg (2.4mg/kg) | Drug and Chemical Toxicology. Vol. 6, Pg. 21, 1983. | |
| mouse | LDLo | intratracheal | 2400ug/kg (2.4mg/kg) | Toxicology Letters. Vol. 30, Pg. 63, 1986. | |
| (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-(methyloxy)-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside | (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside | (1S,3S)-3-Glycoloyl-1,2,3,4,6,11-hexahydro-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1-naphthacenyl-(3-amino-2,3,6-tridesoxy-alpha-L-lyxo-hexopyranosid) |
| (1S,3S)-3-glycoloyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside | (1s,3s)-3,5,12-Trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranoside | (7S,9R)-7-[(2S,4S,5S,6S)-4-Amino-5-hydroxy-6-methyl-oxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione |
| (7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione | (7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-9-glycoloyl-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-quinone;hydrochloride | (7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride |
| (7S,9S)-7-[(2R,4S,5S,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-4-methoxy-6,9,11-tris(oxidanyl)-9-(2-oxidanylethanoyl)-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride | (7S,9S)-7-[[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-9-(2-hydroxy-1-oxoethyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride | (8S,10S)-10-(((2R,4S,5S,6S)-4-Amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracen |
| (8S,10S)-10-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione | (8S,10S)-10-((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione | (8S,10S)-10-((3-Amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione |
| (8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione | (8S-cis)-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-5,12-naphthacenedione | (8S-cis)-10-(3-Amino-2,3,6-Tr ideoxy-alpha-L-Lyxo-Hexopyranosyl)Oxy-7,8,9,10-Tetrahydro-6,8,11-Trihydroxy-8-(Hydroxyacetyl)-1-Methoxy-5,12-Naphthacenedione |
| (8S-cis)-10-(3-Amino-2,3,6-Trideoxy-alpha-L-Lyxo-Hexopyranosyl)Oxy-7,8,9,10-Tetrahydro-6,8,11-Trihydroxy-8-(Hydroxyacetyl)-1-Methoxy-5,12-Naphthacenedione | (8S-cis)-10-[(3-Amino-2,3,6-trideoxy-.alpha.-L-lyxo-hexopyranosyl]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-5,12-naphthacenedione | 1,2,3,4,6,11-Hexahydro-4beta,5,12-trihydroxy-4-(hydroxyacetyl)-10-methoxy-6,11-dioxonaphthacen-1beta-yl-3-amino-2,3,6-trideoxy-alpha-L-lyxohexopyranoside |
| 10-((3-Amino-2,3,6-trideoxy-D-lyxohexopyranosyl)oxy)-8-glycolcyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione | 10-((3-Amino-2,3,6-trideoxy-alpha-L-lyso-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-5,12-naphthacenedione | 10336-EP2270008A1 |
| 10336-EP2270014A1 | 10336-EP2270018A1 | 10336-EP2270505A1 |
| 10336-EP2272827A1 | 10336-EP2272832A1 | 10336-EP2275420A1 |
| 10336-EP2277565A2 | 10336-EP2277566A2 | 10336-EP2277567A1 |
| 10336-EP2277568A2 | 10336-EP2277569A2 | 10336-EP2277570A2 |
| 10336-EP2277865A1 | 10336-EP2277876A1 | 10336-EP2280012A2 |
| 10336-EP2281815A1 | 10336-EP2289892A1 | 10336-EP2292280A1 |
| 10336-EP2292614A1 | 10336-EP2292615A1 | 10336-EP2292617A1 |
| 10336-EP2295055A2 | 10336-EP2295416A2 | 10336-EP2295426A1 |
| 10336-EP2295427A1 | 10336-EP2298748A2 | 10336-EP2298764A1 |
| 10336-EP2298765A1 | 10336-EP2298768A1 | 10336-EP2298778A1 |
| 10336-EP2298780A1 | 10336-EP2301536A1 | 10336-EP2301538A1 |
| 10336-EP2301928A1 | 10336-EP2301933A1 | 10336-EP2302382A2 |
| 10336-EP2302383A2 | 10336-EP2305243A1 | 10336-EP2305250A1 |
| 10336-EP2305640A2 | 10336-EP2305642A2 | 10336-EP2305671A1 |
| 10336-EP2305679A1 | 10336-EP2305689A1 | 10336-EP2308562A2 |
| 10336-EP2308812A2 | 10336-EP2308833A2 | 10336-EP2308855A1 |
| 10336-EP2308861A1 | 10336-EP2311453A1 | 10336-EP2311455A1 |
| 10336-EP2311808A1 | 10336-EP2311825A1 | 10336-EP2311827A1 |
| 10336-EP2311829A1 | 10336-EP2311840A1 | 10336-EP2311842A2 |
| 10336-EP2316452A1 | 10336-EP2316832A1 | 10336-EP2316833A1 |
| 10336-EP2316834A1 | 10336-EP2371811A2 | 10336-EP2374454A1 |
| 13171-EP2269989A1 | 13171-EP2269994A1 | 13171-EP2270008A1 |
| 13171-EP2270018A1 | 13171-EP2270505A1 | 13171-EP2275102A1 |
| 13171-EP2277865A1 | 13171-EP2277876A1 | 13171-EP2281815A1 |
| 13171-EP2286812A1 | 13171-EP2289892A1 | 13171-EP2292227A2 |
| 13171-EP2292233A2 | 13171-EP2292614A1 | 13171-EP2292615A1 |
| 13171-EP2292617A1 | 13171-EP2295407A1 | 13171-EP2295412A1 |
| 13171-EP2295413A1 | 13171-EP2295416A2 | 13171-EP2298305A1 |
| 13171-EP2298736A1 | 13171-EP2298746A1 | 13171-EP2298748A2 |
| 13171-EP2298768A1 | 13171-EP2298772A1 | 13171-EP2298780A1 |
| 13171-EP2301933A1 | 13171-EP2305640A2 | 13171-EP2305642A2 |
| 13171-EP2305671A1 | 13171-EP2305679A1 | 13171-EP2305689A1 |
| 13171-EP2308833A2 | 13171-EP2308839A1 | 13171-EP2308861A1 |
| 13171-EP2311827A1 | 13171-EP2316832A1 | 13171-EP2316833A1 |
| 13171-EP2316834A1 | 13171-EP2316937A1 | 14-Hydroxydaunomycin |
| 14-Hydroxydaunorubicine | 14D928 | 23214-92-8 |
| 45970-EP2275413A1 | 45970-EP2287156A1 | 45970-EP2311807A1 |
| 5,12-Naphthacenedione, 10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S,10S)- | 5,12-Naphthacenedione, 10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S-cis)- | 5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-, (8S,10S)- |
| 80168379AG | A14403 | AB1009243 |
| ADM | ADR | AKOS015951330 |
| AOJJSUZBOXZQNB-TZSSRYMLSA-N | Adriablastin | Adriacin (hydrochloride salt) |
| Adriamycin | Adriamycin | Adriamycin PFS (hydrochloride salt) |
| Adriamycin RDF (hydrochloride salt) | Adriamycin semiquinone | Adriblas tina |
| Adriblastina | Adriblastina (TN) | Adriblastina (hydrochloride salt) |
| Aerosolized Doxorubicin | BDBM22984 | BDBM32022 |
| BP-23114 | BPBio1_000502 | BRD-K92093830-003-04-3 |
| BRD-K92093830-003-25-8 | BSPBio_000456 | BSPBio_001031 |
| C-23275 | C01661 | CC-23807 |
| CCRIS 739 | CHEBI:28748 | CHEMBL53463 |
| CS-2759 | Caelyx (liposomal doxorubicin) | Conjugate of doxorubicin with humanized monoclonal antibody LL1 against CD74 |
| Conjugate of doxorubicin with monoclonal antibody P4/D10 against GP120 | D03899 | DB00997 |
| DOX | DOX-SL | DTXSID8021480 |
| Doxil | Doxorubicin | Doxorubicin (USAN/INN) |
| Doxorubicin [USAN:INN:BAN] | Doxorubicin hydrochloride (hydrochloride salt) | Doxorubicin-P4/D10 |
| Doxorubicin-P4/D10 conjugate | Doxorubicin-hLL1 | Doxorubicin-hLL1 conjugate |
| Doxorubicina | Doxorubicina [INN-Spanish] | Doxorubicine |
| Doxorubicine [INN-French] | Doxorubicinum | Doxorubicinum [INN-Latin] |
| EINECS 245-495-6 | FI 106 | FT-0601614 |
| Farmablastina (hydrochloride salt) | GR-319 | GTPL7069 |
| HMS2089H06 | HSDB 3070 | HY-15142A |
| Hydroxydaunomycin hydrochlor ide (hydrochloride salt) | Hydroxydaunomycin hydrochloride (hydrochloride salt) | Hydroxydaunorubicin |
| Hydroxydaunorubicin hydrochloride (hydrochloride salt) | LMPK13050001 | LS-165655 |
| MCULE-4188577717 | MLS000028393 | NCGC00024415-35 |
| NCGC00024415-37 | NCGC00024415-38 | NCGC00024415-40 |
| NCGC00024415-41 | NCGC00024415-42 | NCI-C01514 |
| NDC 38242-874 | Prestwick0_000438 | Prestwick1_000438 |
| Prestwick2_000438 | Prestwick3_000438 | Probes1_000151 |
| Probes2_000129 | Q18936 | Rubex (hydrochloride salt) |
| SB21799 | SCHEMBL3243 | SMP1_000106 |
| SPBio_002395 | TLC D-99 | ThermoDox |
| UNII-80168379AG | ZINC3918087 | adiblastine (hydrochloride salt) |
| adr iablatina (hydrochloride salt) | adriablastine (hydrochloride salt) | adriablatina (hydrochloride salt) |
| adriblatina (hydrochloride salt) | cid_443939 | doxorubicin |
| DrugBank Name | Doxorubicin |
| DrugBank | DB00997 |
| CAS Number | 111266-55-8, 23214-92-8, 25316-40-9 |
| PubChem Compound | 31703 |
| KEGG Compound ID | C01661 |
| KEGG Drug | D03899 |
| PubChem.Substance | 46507641 |
| ChEBI | 28748 |
| PharmGKB | PA449412 |
| ChemSpider | 29400 |
| BindingDB | 22984.0 |
| TTD | DNC000163 |
| Wikipedia | Doxorubicin |
| HET | DM2 |
| DPD | 2298 |