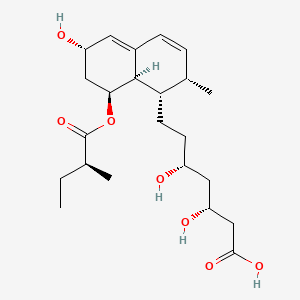
D0442 | pravastatin
C
C10BX02 Pravastatin and acetylsalicylic acid
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BA03 Pravastatin and fenofibrate
[C10BA] HMG CoA reductase inhibitors in combination with other lipid modifying agents
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10AA03 Pravastatin
[C10AA] HMG CoA reductase inhibitors
[C10A] LIPID MODIFYING AGENTS, PLAIN
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| OPENING OF PERMEABILITY TRANSITION PORE (PTP) | >100 | 1 hour | Human | HepG2 | High-content screening assay | Negative | MEC | 306 |
| MEMBRANE POTENTIAL | > 200 µM | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | decrease | EC20 | 36 |
| MEMBRANE POTENTIAL | >100 µM | 1 hour | Human | HepG2 | High-content screening assay | Negative | MEC | 306 |
| RESPIRATION | 5 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | decrease | EC20 | 36 |
| RESPIRATION | ND | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | Negative | EC20 | 36 |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex I activity | Negative | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | Negative | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | Negative | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex IV activity | Negative | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex V activity | Negative | p < 0.05 | 3 | |
| SWELLING | > 200 µM | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | increase | EC20 | 36 |
| ROS PRODUCTION | 50 µM | 1 hour | Human | HepG2 | High-content screening assay | Increase | MEC | 306 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 50 μM | bovine | heart mitochondria | Measurement of complex I activity | Negative | p < 0.05 | 3 | |
| NADH:ubiquinone reductase | 5 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | inhibit | EC20 | 36 |
| Succinate dehydrogenase | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | Negative | p < 0.05 | 3 | |
| Succinate dehydrogenase | ND | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | Negative | EC20 | 36 |
| Quinol--cytochrome-c reductase | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | Negative | p < 0.05 | 3 | |
| Cytochrome c oxidase | 50 μM | bovine | heart mitochondria | Measurement of complex IV activity | Negative | p < 0.05 | 3 | |
| ATP synthase | 50 μM | bovine | heart mitochondria | Measurement of complex V activity | Negative | p < 0.05 | 3 | |
| Reactive oxygen species | 50 µM | 1 hour | Human | HepG2 | High-content screening assay | increase | MEC | 306 |
| Cytochrome c | > 200 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 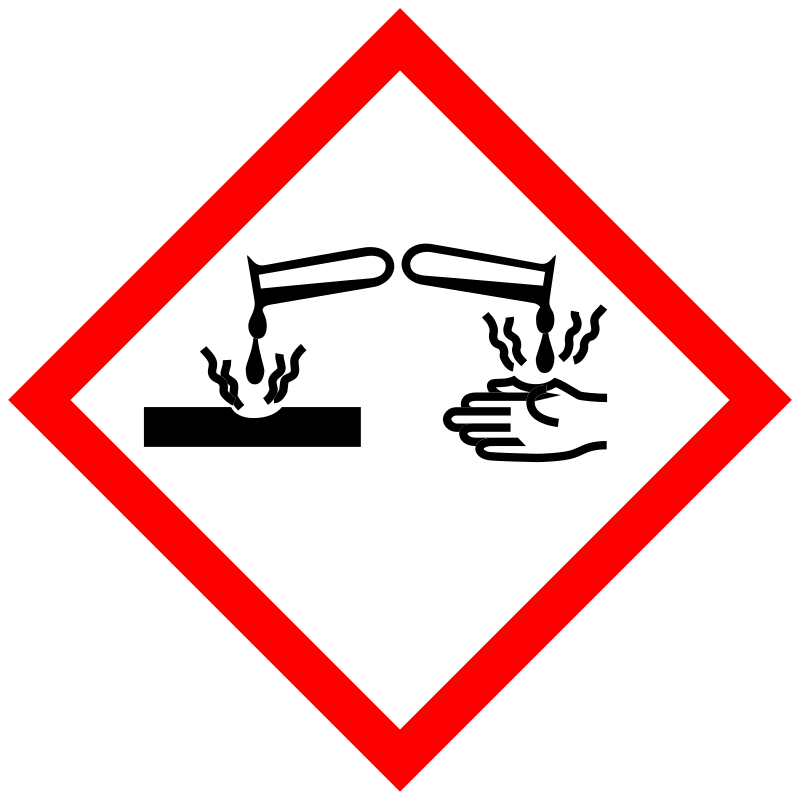  |
Danger |
Aggregated GHS information provided by 9 companies from 1 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H228 (100%): Flammable solid [Danger Flammable solids] H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H411 (100%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P210, P240, P241, P260, P264, P273, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P370+P378, P391, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | subcutaneous | 3172mg/kg (3172mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 15, Pg. 4949, 1987. | |
| mouse | LD50 | oral | 8939mg/kg (8939mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 15, Pg. 4949, 1987. | |
| women | TDLo | oral | 30mg/kg/21W-I (30mg/kg) | New England Journal of Medicine. Vol. 327, Pg. 649, 1992. | |
| rat | LD50 | oral | > 12gm/kg (12000mg/kg) | Yakkyoku. Pharmacy. Vol. 40, Pg. 2351, 1989. | |
| man | TDLo | oral | 16mg/kg/8W-I (16mg/kg) | American Journal of Emergency Medicine. Vol. 17, Pg. 1388, 1999. | |
| mouse | LD50 | subcutaneous | 2975mg/kg (2975mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 15, Pg. 4949, 1987. | |
| rat | LDLo | intraperitoneal | 400mg/kg (400mg/kg) | Biochemistry and Molecular Biology International. Vol. 47, Pg. 519, 1999. | |
| mouse | LD50 | intravenous | 2011mg/kg (2011mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 15, Pg. 4949, 1987. | |
| women | TDLo | oral | 16800ug/kg (16.8mg/kg) | behavioral: somnolence (general depressed activity) | Lancet. Vol. 340, Pg. 910, 1992. |
| rat | LD50 | intravenous | 440mg/kg (440mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 15, Pg. 4949, 1987. | |
| (+)-(3R,5R)-3,5-dihydroxy-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-{[(S)-2-methylbutyryl]oxy}-1,2,6,7,8,8a-hexahydro-1-naphthyl]heptanoic acid | (3R,5R)-3,5-dihydroxy-7-((1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-((S)-2-methylbutanoyloxy)-1,2,6,7,8,8a-hexahydronaphthalen-1-yl)heptanoic acid; | (3R,5R)-3,5-dihydroxy-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-{[(2S)-2-methylbutanoyl]oxy}-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]heptanoic acid |
| (3R,5R)-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-[(2S)-2-methylbutanoyl]oxy-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-dihydroxyheptanoic acid | (3R,5R)-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-{[(2S)-2-methylbutanoyl]oxy}-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-dihydroxyheptanoic acid | 093P370 |
| 1,2,6,7,8,8a-hexahydro-beta,delta,6-trihydroxy-2-methyl-8-(2-methyl-1-oxobutoxy)-, (1S-(1alpha(betaS*,deltaS*),2alpha,6alpha,8beta(R*),8aalpha))-1-Naphthaleneheptanoic acid | 1-Naphthaleneheptanoic acid, 1,2,6,7,8,8a-hexahydro-beta,delta,6-trihydroxy-2-methyl-8-((2S)-2-methyl-1-oxobutoxy)-, (betaR,deltaR,1S,2S,6S,8S,8aR)- | 1-Naphthaleneheptanoic acid, 1,2,6,7,8,8a-hexahydro-beta,delta,6-trihydroxy-2-methyl-8-(2-methyl-1-oxobutoxy)-, (1S-(1alpha(betas*,deltas*),2alpha,6alpha,8beta(R*),8aalpha))- |
| 3beta-Hydroxycompactin | 81093-37-0 | ACT02637 |
| AKOS015895229 | BDBM20688 | BIDD:GT0773 |
| BRD-K60511616-236-01-4 | BRD-K60511616-236-02-2 | BRD-K60511616-236-08-9 |
| C-22133 | C01844 | C10AA03 |
| C23H36O7 | CC-33900 | CCG-221195 |
| CCRIS 7557 | CHEBI:63618 | CHEMBL1144 |
| D08410 | DB00175 | DTXSID6023498 |
| Eptastatin | FT-0082682 | GTPL2953 |
| HMS3715P11 | HSDB 8368 | HY-B0165 |
| KS-5015 | KXO2KT9N0G | LMFA05000695 |
| LS-94713 | Mevalothin | NCGC00188962-01 |
| NCGC00188962-02 | PRAVASTATIN SODIUM | Pravachol |
| Pravastatin (INN) | Pravastatin [INN:BAN] | Pravastatin acid |
| Pravastatin tert-Octylamine Salt | Pravastatina | Pravastatina [Spanish] |
| Pravastatine | Pravastatine [French] | Pravastatinum |
| Pravastatinum [Latin] | Pravator (TN) | Q1240093 |
| RMS-431 | SC-17346 | SCHEMBL1117 |
| SQ-31,000 | SQ-31000 | SR-01000781259 |
| SR-01000781259-2 | TUZYXOIXSAXUGO-PZAWKZKUSA-N | UNII-KXO2KT9N0G |
| ZINC3798763 | pravastatin | s5713 |
| DrugBank Name | pravastatin |
| DrugBank | DB00175 |
| CAS Number | 115873-26-2, 81093-37-0 |
| PubChem Compound | 54687 |
| KEGG Compound ID | C01844 |
| KEGG Drug | D08410 |
| PubChem.Substance | 46504851 |
| ChEBI | 20688 |
| PharmGKB | PA451089 |
| ChemSpider | 49398 |
| BindingDB | 20688.0 |
| TTD | DAP000550 |
| Wikipedia | Pravastatin |