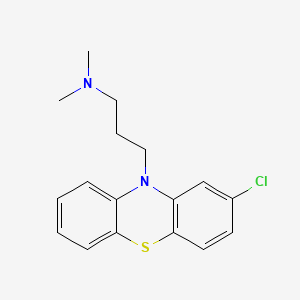
D0024 | Chlorpromazine
N
N05AA01 Chlorpromazine
[N05AA] Phenothiazines with aliphatic side-chain
[N05A] ANTIPSYCHOTICS
[N05] PSYCHOLEPTICS
[N] Nervous system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| OPENING OF PERMEABILITY TRANSITION PORE (PTP) | >100 µM | 1 hour | Human | HepG2 | High-content screening assay | Affect | MEC | 306 |
| ELECTROPHORETIC UNCOUPLING | 278 | |||||||
| MEMBRANE POTENTIAL | 100 µM | 1 hour | Human | HepG2 | High-content screening assay | Decrease | MEC | 306 |
| STATE 3 RESPIRATION | decrease | 22 | ||||||
| STATE 4 RESPIRATION | increase | 22 | ||||||
| OXYGEN CONSUMPTION RATE (OCR) | > 300 μM | 2 minutes | human | HepG2 | Measurement of OCR | increase | EC50 | 7 |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex I activity | Negative | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | decrease | p < 0.001 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | decrease | p < 0.001 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex IV activity | Negative | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex V activity | decrease | p < 0.001 | 3 | |
| ELECTRON TRANSPORT CHAIN | rat | isolated liver mitochondria | decrease | 23 | ||||
| ELECTRON TRANSPORT CHAIN | decrease | 7 | ||||||
| ELECTRON TRANSPORT CHAIN | inhibit | 197 | ||||||
| ECAR | > 300 μM | 2 minutes | human | HepG2 | Measurement of ECAR | increase | EC50 | 7 |
| GLUCOSE GALACTOSE IC50 RATIO | 36.6 ± 19.4, 48.4 ± 11.9, 0.8, 17.3 ± 7.6, 25.3 ± 10.8, 0.7 | 4hr | H9c2 cells | high-glucose–galactose cell viability assay with JC-1 mitochondrial membrane potential and ATP-depletion assays (CellTiter-Glo reagent ). | glucose/galactose IC50 ratio (JC-1 IC50 in glucose, JC-1 IC50 in galactose, JC-1 glu/gla, ATP IC50 in glucose, ATP IC50 in galactose, ATP glu/gla ) | 50 | ||
| POTASSIUM RELEASE | increase | 22 | ||||||
| ROS PRODUCTION | 50 µM | 1 hour | Human | HepG2 | High-content screening assay | Increase | MEC | 306 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 50 μM | bovine | heart mitochondria | Measurement of complex I activity | Negative | p < 0.05 | 3 | |
| NADH:ubiquinone reductase | rat | isolated liver mitochondria | inhibitor | 23 | ||||
| NADH:ubiquinone reductase | inhibitor | 7 | ||||||
| Succinate dehydrogenase | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | inhibitor | p < 0.001 | 3 | |
| Quinol--cytochrome-c reductase | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | inhibitor | p < 0.001 | 3 | |
| Cytochrome c oxidase | 50 μM | bovine | heart mitochondria | Measurement of complex IV activity | Negative | p < 0.05 | 3 | |
| ATP synthase | 50 μM | bovine | heart mitochondria | Measurement of complex V activity | inhibitor | p < 0.001 | 3 | |
| potassium | not specified | 22 | ||||||
| Reactive oxygen species | 50 µM | 1 hour | Human | HepG2 | High-content screening assay | increase | MEC | 306 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
   |
Danger |
Aggregated GHS information provided by 2 companies from 1 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H301 (100%): Toxic if swallowed [Danger Acute toxicity, oral] H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin] H331 (100%): Toxic if inhaled [Danger Acute toxicity, inhalation] H362 (100%): May cause harm to breast-fed children [Reproductive toxicity, effects on or via lactation] H400 (100%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (100%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P260, P261, P263, P264, P270, P271, P272, P273, P280, P301+P310, P302+P352, P304+P340, P308+P313, P311, P321, P330, P333+P313, P363, P391, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | > 2gm/kg (2000mg/kg) | Antibiotics Annual. Vol. 3, Pg. 534, 1955/1956. | |
| monkey | LD50 | subcutaneous | > 5mg/kg (5mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 11, Pg. 932, 1961. | |
| rabbit | LD50 | intravenous | 16mg/kg (16mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 120, Pg. 450, 1959. | |
| rat | LD50 | intramuscular | > 2gm/kg (2000mg/kg) | Antibiotics Annual. Vol. 3, Pg. 534, 1955/1956. | |
| rabbit | LD50 | intravenous | 5mg/kg (5mg/kg) | "Psychotropic Drugs and Related Compounds," 2nd ed., Usdin, E., and D.H. Efron, Washington, DC, 1972Vol. -, Pg. 15, 1972. | |
| mammal (species unspecified) | LD50 | oral | 500mg/kg (500mg/kg) | Journal of Medicinal Chemistry. Vol. 8, Pg. 836, 1965. | |
| rat | LD50 | intravenous | 243mg/kg (243mg/kg) | Antibiotics Annual. Vol. 3, Pg. 534, 1955/1956. | |
| rat | LD50 | intraperitoneal | 137mg/kg (137mg/kg) | German Offenlegungsschrift Patent Document. Vol. #2350222, | |
| guinea pig | LD50 | subcutaneous | 420mg/kg (420mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 137, Pg. 375, 1962. | |
| mouse | LC50 | inhalation | 40mg/m3/2H (40mg/m3) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 1106, 1986. | |
| women | TDLo | oral | 35gm/kg/16Y-I (35000mg/kg) | Biological Psychiatry. Vol. 18, Pg. 1441, 1983. | |
| mouse | LD50 | intramuscular | > 2gm/kg (2000mg/kg) | Antibiotics Annual. Vol. 3, Pg. 534, 1955/1956. | |
| rat | LD50 | subcutaneous | 75mg/kg (75mg/kg) | Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 56, Pg. 377, 1960. | |
| guinea pig | LD50 | intraperitoneal | 87mg/kg (87mg/kg) | Medicina Experimentalis. Vol. 8, Pg. 237, 1963. | |
| women | TDLo | oral | 6mg/kg (6mg/kg) | Postgraduate Medical Journal. Vol. 60, Pg. 564, 1984. | |
| mouse | LD50 | intravenous | 20mg/kg (20mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 21, Pg. 808, 1971. | |
| mouse | LD50 | intravenous | 16mg/kg (16mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 11, Pg. 932, 1961. | |
| mouse | LD50 | oral | 135mg/kg (135mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 118, Pg. 358, 1959. | |
| women | TDLo | oral | 16800ug/kg/3W (16.8mg/kg) | blood: agranulocytosis | Postgraduate Medical Journal. Vol. 69, Pg. 885, 1993. |
| rat | LD50 | intravenous | 25mg/kg (25mg/kg) | Fortschritte der Arzneimittelforschung. Progress in Drug Research. Vol. 5, Pg. 269, 1963. | |
| rat | LD50 | oral | 145mg/kg (145mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 148, Pg. 151, 1965. | |
| mouse | LDLo | intracrebral | 2mg/kg (2mg/kg) | behavioral: convulsions or effect on seizure threshold | Planta Medica. Vol. 49, Pg. 103, 1983. |
| mouse | LD50 | subcutaneous | 420mg/kg (420mg/kg) | British Journal of Pharmacology and Chemotherapy. Vol. 22, Pg. 301, 1964. | |
| chicken | LD50 | intravenous | 28mg/kg (28mg/kg) | Toxicology and Applied Pharmacology. Vol. 2, Pg. 558, 1960. | |
| guinea pig | LDLo | oral | 250mg/kg (250mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 11, Pg. 561, 1976. | |
| rat | LC50 | inhalation | 40mg/m3/2H (40mg/m3) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 1106, 1986. | |
| guinea pig | LD50 | intraperitoneal | 109mg/kg (109mg/kg) | Pharmazie. Vol. 38, Pg. 749, 1983. | |
| mouse | LD50 | oral | 6257mg/kg (6257mg/kg) | behavioral: excitement | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 125, Pg. 83, 1960. |
| women | TDLo | oral | 240mg/kg/30D- (240mg/kg) | Journal of Clinical Psychiatry. Vol. 46, Pg. 341, 1985. | |
| guinea pig | LD50 | intraperitoneal | 374mg/kg (374mg/kg) | Antibiotics Annual. Vol. 3, Pg. 534, 1955/1956. | |
| monkey | LDLo | oral | > 30mg/kg (30mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 122, Pg. 129, 1959. | |
| mouse | LD50 | intraperitoneal | 14mg/kg (14mg/kg) | Farmaco, Edizione Scientifica. Vol. 14, Pg. 269, 1959. | |
| mouse | LD50 | unreported | 82500ug/kg (82.5mg/kg) | Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 53, Pg. 2S, 1957. | |
| rat | LC50 | inhalation | 209mg/m3/2H (209mg/m3) | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 10, Pg. 73, 1968. | |
| dog | LD50 | intravenous | 30mg/kg (30mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 118, Pg. 358, 1959. | |
| rabbit | LD50 | oral | 5848mg/kg (5848mg/kg) | Antibiotics and Chemotherapy Vol. 10, Pg. 376, 1960. | |
| man | TDLo | oral | 217mg/kg/19D (217mg/kg) | Journal of Clinical Psychiatry. Vol. 46, Pg. 341, 1985. | |
| rat | LD50 | oral | 142mg/kg (142mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 18, Pg. 261, 1968. | |
| women | TDLo | oral | 200ug/kg (0.2mg/kg) | New York State Journal of Medicine. Vol. 57, Pg. 1922, 1957. | |
| infant | TDLo | oral | 20mg/kg (20mg/kg) | American Journal of Diseases of Children. Vol. 130, Pg. 507, 1976. | |
| child | LDLo | oral | 37500ug/kg (37.5mg/kg) | New England Journal of Medicine. Vol. 256, Pg. 527, 1957. | |
| women | TDLo | oral | 166mg/kg/18D- (166mg/kg) | Postgraduate Medical Journal. Vol. 53, Pg. 278, 1977. | |
| women | TDLo | oral | 240mg/kg/30D (240mg/kg) | Journal of Clinical Psychiatry. Vol. 46, Pg. 341, 1985. | |
| man | TDLo | oral | 8900ug/kg (8.9mg/kg) | lungs, thorax, or respiration: dyspnea | American Journal of Emergency Medicine. Vol. 14, Pg. 467, 1996. |
| women | TDLo | intravenous | 822ug/kg (0.822mg/kg) | Annals of Emergency Medicine. Vol. 17, Pg. 380, 1988. | |
| cat | LD50 | subcutaneous | > 10mg/kg (10mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 11, Pg. 932, 1961. | |
| man | TDLo | intravenous | 1786ug/kg/2D- (1.786mg/kg) | Biological Psychiatry. Vol. 18, Pg. 1441, 1983. | |
| rat | LD50 | intraperitoneal | > 2gm/kg (2000mg/kg) | Antibiotics Annual. Vol. 3, Pg. 534, 1955/1956. | |
| mouse | LC50 | inhalation | 209mg/m3/2H (209mg/m3) | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 10, Pg. 73, 1968. | |
| chicken | LD50 | intraperitoneal | 160mg/kg (160mg/kg) | Toxicology and Applied Pharmacology. Vol. 2, Pg. 558, 1960. | |
| rat | LD50 | oral | 8900mg/kg (8900mg/kg) | Antibiotics and Chemotherapy Vol. 12, Pg. 249, 1962. | |
| guinea pig | LD50 | oral | 382mg/kg (382mg/kg) | Antibiotics and Chemotherapy Vol. 11, Pg. 276, 1961. | |
| dog | LDLo | oral | 250mg/kg (250mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 11, Pg. 561, 1976. | |
| mouse | LDLo | intramuscular | 300mg/kg (300mg/kg) | Therapie. Vol. 15, Pg. 1064, 1960. | |
| mouse | LD50 | oral | 135mg/kg (135mg/kg) | Chemical and Pharmaceutical Bulletin. Vol. 24, Pg. 1179, 1976. | |
| human | TDLo | oral | 8570ug/kg/12D (8.57mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 93, 1972. | |
| man | TDLo | oral | 6071ug/kg (6.071mg/kg) | Israel Journal of Medical Sciences. Vol. 5, Pg. 1254, 1969. | |
| mouse | LD50 | subcutaneous | 33mg/kg (33mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 11, Pg. 932, 1961. | |
| rat | LD50 | intravenous | 23mg/kg (23mg/kg) | Farmaco, Edizione Pratica. Vol. 26, Pg. 585, 1971. | |
| cat | LDLo | oral | 100mg/kg (100mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 11, Pg. 561, 1976. | |
| dog | LD50 | subcutaneous | > 20mg/kg (20mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 11, Pg. 932, 1961. | |
| rat | LD50 | unreported | 90mg/kg (90mg/kg) | "Psychotropic Drugs and Related Compounds," 2nd ed., Usdin, E., and D.H. Efron, Washington, DC, 1972Vol. -, Pg. 15, 1972. | |
| rat | LD50 | subcutaneous | 11250mg/kg (11250mg/kg) | gastrointestinal: "hypermotility, diarrhea" | Toxicology and Applied Pharmacology. Vol. 9, Pg. 445, 1966. |
| mouse | LD50 | intraperitoneal | 92200ug/kg (92.2mg/kg) | Toxicology and Applied Pharmacology. Vol. 2, Pg. 558, 1960. | |
| mouse | LD50 | intravenous | 240mg/kg (240mg/kg) | Antibiotics Annual. Vol. 3, Pg. 534, 1955/1956. | |
| guinea pig | LD50 | intravenous | 303mg/kg (303mg/kg) | Research Progress in Organic-Biological and Medicinal Chemistry. Vol. 2, Pg. 306, 1970. | |
| rat | LD50 | intraperitoneal | 62mg/kg (62mg/kg) | Psychopharmacologia Vol. 12, Pg. 142, 1968. | |
| guinea pig | LD50 | intramuscular | 1180mg/kg (1180mg/kg) | Antibiotics Annual. Vol. 3, Pg. 534, 1955/1956. | |
| man | TDLo | oral | 18mg/kg (18mg/kg) | Israel Journal of Medical Sciences. Vol. 5, Pg. 1254, 1969. | |
| (chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine | (chlorpromazine)[3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine | 10-(3-Dimethylaminopropyl)-2-chlorophenothiazine |
| 10H-Phenothiazine-10-propanamine, 2-chloro-N,N-dimethyl- | 10H-Phenothiazine-10-propanamine, 2-chloro-N,N-dimethyl-, radical ion(1+) | 1N,1N-dimethyl-3-(2-chloro-10H-10-phenothiazinyl)-1-propanamine |
| 2-Chloro-10-(3-(dimethylamino)propyl)phenothiazine | 2-Chloro-10-(3-dimethylaminopropyl)phenothiazine | 2-Chloro-10-[3-(dimethylamino)propyl]phenothiazine |
| 2-Chloropromazine | 2-Cloro-10 (3-dimetilaminopropil)fenotiazina | 2-Cloro-10 (3-dimetilaminopropil)fenotiazina [Italian] |
| 2-chloro-10-(3-(dimethylamino)propyl)-phenothiazine | 2-chloro-10-[3-(dimethylamino)propyl]- | 2-chloro-N,N-dimethyl-10H-Phenothiazine-10-propanamine |
| 2601-A | 2601A | 3-(2-Chloro-10H-phenothiazin-10-yl)-N,N-dimethyl-1-propanamine |
| 3-(2-Chloro-10H-phenothiazin-10-yl)-N,N-dimethyl-1-propanamine # | 3-(2-chloro-10H-phenothiazin-10-yl)-N,N-dimethylpropan-1-amine | 3-(2-chlorophenothiazin-10-yl)-N,N-dimethyl-propan-1-amine |
| 3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine | 34468-21-8 | 4560 R.P |
| 4560 R.P. | 4560 Rp hydrochloride | 50-53-3 |
| AB00051943 | AB00051943-15 | AB00051943-16 |
| AB00051943_17 | AB00051943_18 | AB0015094 |
| AKOS001490972 | ALBB-022464 | API0001970 |
| AX8120990 | Aminasine | Aminazin |
| Aminazine | Ampliactil | Amplicitil |
| Amplictil | BBL028251 | BC 135 |
| BCP03610 | BDBM50001888 | BPBio1_000273 |
| BPBio1_001181 | BRD-K89997465-001-05-3 | BSPBio_000247 |
| BSPBio_002011 | Bio1_000457 | Bio1_000946 |
| Bio1_001435 | Biomol-NT_000020 | C06906 |
| C17H19ClN2S | CAS-50-53-3 | CAS-69-09-0 |
| CCG-40059 | CCRIS 3711 | CHEBI:3647 |
| CHEMBL71 | CPZ | CTK1C5154 |
| Chlor-PZ | Chlor-Promanyl | Chlordelazin |
| Chlordelazine | Chlorderazin | Chloro-3 (dimethylamino-3 propyl)-10 phenothiazine |
| Chloro-3 (dimethylamino-3 propyl)-10 phenothiazine [French] | Chloropromazine | Chlorpromados |
| Chlorpromazin | Chlorpromazine (USP/INN) | Chlorpromazine Tannate |
| Chlorpromazine [USAN:INN:BAN] | Chlorpromazine [USP:INN:BAN] | Chlorpromazine cation radical |
| Chlorpromazine;[3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine | Chlorpromazinum | Chlorpromazinum [INN-Latin] |
| Chlropromados | Clorpromazina | Clorpromazina [INN-Spanish] |
| Clorpromazina [Italian] | Contomin | Cromedazine |
| D00270 | DB00477 | DSSTox_CID_2808 |
| DSSTox_GSID_22808 | DSSTox_RID_76736 | DTXSID0022808 |
| DivK1c_000624 | EINECS 200-045-8 | Elmarin |
| Epitope ID:136898 | Esmind | FT-0653683 |
| Fenactil | Fenaktyl | Fraction |
| Fraction AB | GG 407 | GTPL83 |
| HL 5746 | HMS1920M03 | HMS2089C12 |
| HMS2091E06 | HMS3430C19 | HMS501P06 |
| HSDB 3033 | Hibanil | Hibernal (Salt/Mix) |
| IDI1_000624 | J10069 | JHICC02042 |
| KBio1_000624 | KBio2_000622 | KBio2_002312 |
| KBio2_003190 | KBio2_004880 | KBio2_005758 |
| KBio2_007448 | KBio3_001231 | KBio3_002792 |
| KBioGR_000806 | KBioGR_002312 | KBioSS_000622 |
| KBioSS_002314 | KS-00002WW5 | KS-5101 |
| KSC-315-032- | KUC112482N | L000182 |
| LS-105361 | Largactil | Largactilothiazine |
| Largactyl | Lomazine (Salt/Mix) | Lopac-C-8138 |
| Lopac0_000249 | M-1209 | M176 |
| MCULE-5976054678 | MLS003166901 | Megaphen |
| N-(3-Dimethylaminopropyl)-3-chlorophenothiazine | NCGC00015273-01 | NCGC00015273-02 |
| NCGC00015273-03 | NCGC00015273-04 | NCGC00015273-05 |
| NCGC00015273-06 | NCGC00015273-07 | NCGC00015273-08 |
| NCGC00015273-09 | NCGC00015273-10 | NCGC00015273-11 |
| NCGC00015273-12 | NCGC00015273-13 | NCGC00015273-14 |
| NCGC00015273-15 | NCGC00015273-16 | NCGC00015273-17 |
| NCGC00015273-19 | NCGC00024409-04 | NCGC00024409-05 |
| NCGC00024409-06 | NCGC00024409-07 | NCGC00024409-08 |
| NINDS_000624 | NSC 167745 | NSC-167745 |
| NSC-756689 | NSC167745 | NSC17479 |
| NSC756689 | Norcozine (Salt/Mix) | Novomazina |
| Oprea1_110255 | Pharmakon1600-01500184 | Phenactyl |
| Phenathyl | Phenothiazine hydrochloride | Phenothiazine, 2-chloro-10-(3-(dimethylamino)propyl)- |
| Phenothiazine, 2-chloro-10-[3-(dimethylamino)propyl]- | Plegomasine | Prazilpromactil |
| Prestwick0_000064 | Prestwick1_000064 | Prestwick2_000064 |
| Prestwick3_000064 | Proma | Promactil |
| Promazil | Propaphen | Propaphenin |
| Prozil | Psychozine | Q407972 |
| QSPL 401 | QTL1_000021 | R1242 |
| RP-4560 | SBI-0050237.P004 | SC-50091 |
| SCHEMBL8321 | SKF 2601-A | SKF 2601A |
| SKF-2601 | SMR001453710 | SPBio_001111 |
| SPBio_002168 | SPECTRUM1500184 | SR-01000000012-5 |
| SR-01000000012-6 | STK182870 | Sanopron |
| Spectrum2_001156 | Spectrum3_000346 | Spectrum4_000283 |
| Spectrum5_000717 | Spectrum_000142 | Thorazine |
| Thorazine (TN) | Thorazine Suppositories | Thorazine hydrochloride |
| Torazina | Tox21_110120 | Tox21_110120_1 |
| Tranzine (Salt/Mix) | U42B7VYA4P | UNII-U42B7VYA4P |
| Unitensen | WLN: T C666 BN ISJ B3N1&1 EG | Wintermin |
| Z80 | ZINC44027 | ZPEIMTDSQAKGNT-UHFFFAOYSA-N |
| [3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine (chlor-promazine) | [3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine( Chlorpromazine) | [3-(2-Chloro-phenothiazin-10-yl)-propyl]-dimethyl-amine(clorpromazine) |
| [3-(2-chloro-10H-phenothiazin-10-yl)propyl]dimethylamine | [3-(2-chloro-10H-phenothiazin-10-yl)propyl]dimethylamine hydrochloride | cMAP_000017 |
| chlorpromazine |
| DrugBank Name | Chlorpromazine |
| DrugBank | DB00477 |
| CAS Number | 113-98-4, 146702-01-4, 34468-21-8, 50-53-3, 69-09-0 |
| PubChem Compound | 2726 |
| KEGG Compound ID | C06906 |
| KEGG Drug | D00270 |
| PubChem.Substance | 46508395 |
| ChEBI | 3647 |
| PharmGKB | PA448964 |
| ChemSpider | 2625 |
| BindingDB | 50001888.0 |
| TTD | DAP000374 |
| Wikipedia | Chlorpromazine |
| HET | Z80 |
| DPD | 8017|20237 |