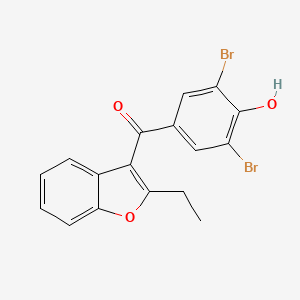
D0419 | benzbromarone
M
M04AB03 Benzbromarone
[M04AB] Preparations increasing uric acid excretion
[M04A] ANTIGOUT PREPARATIONS
[M04] ANTIGOUT PREPARATIONS
[M] Musculoskeletal system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| UNCOUPLING | 0.1 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| UNCOUPLING | 1 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| UNCOUPLING | 10 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | affect | p < 0.01 | 4 | |
| UNCOUPLING | 20 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | affect | p < 0.01 | 4 | |
| UNCOUPLING | 50 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | affect | p < 0.01 | 4 | |
| UNCOUPLING | 80 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | affect | p < 0.01 | 4 | |
| UNCOUPLING | 100 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | affect | p < 0.01 | 4 | |
| UNCOUPLING | increase | 35 | ||||||
| MEMBRANE POTENTIAL | 0.5 μmol/L | 1 hour | rat; Sprague–Dawley | hepatocytes | Measurement of mitochondrial membrane potential | Negative | p < 0.05 | 4 |
| MEMBRANE POTENTIAL | 1 μmol/L | 1 hour | rat; Sprague–Dawley | hepatocytes | Measurement of mitochondrial membrane potential | decrease | p < 0.01 | 4 |
| MEMBRANE POTENTIAL | 4 μmol/L | 1 hour | rat; Sprague–Dawley | hepatocytes | Measurement of mitochondrial membrane potential | decrease | p < 0.01 | 4 |
| MEMBRANE POTENTIAL | 8 μmol/L | 1 hour | rat; Sprague–Dawley | hepatocytes | Measurement of mitochondrial membrane potential | decrease | p < 0.01 | 4 |
| MEMBRANE POTENTIAL | 20 μmol/L | 1 hour | rat; Sprague–Dawley | hepatocytes | Measurement of mitochondrial membrane potential | decrease | p < 0.01 | 4 |
| MEMBRANE POTENTIAL | 7.22±1.24 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 13.77 | human | HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 4.91±1.79 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| STATE 3 RESPIRATION | 0.1 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| STATE 3 RESPIRATION | 1 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| STATE 3 RESPIRATION | 10 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| STATE 3 RESPIRATION | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| STATE 3 RESPIRATION | 0.1 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| STATE 3 RESPIRATION | 1 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| STATE 3 RESPIRATION | 10 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| STATE 3 RESPIRATION | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 0.1 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 1 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 10 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 0.1 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 1 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 10 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| ELECTRON TRANSPORT CHAIN | decrease | 37 | ||||||
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 0.5 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of beta oxidation and ketone body formation | Negative | p < 0.05 | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 2 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of beta oxidation and ketone body formation | decrease | p < 0.05 | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 5 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of beta oxidation and ketone body formation | decrease | p < 0.01 | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 10 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of beta oxidation and ketone body formation | decrease | p < 0.01 | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 50 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of beta oxidation and ketone body formation | decrease | p < 0.01 | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of acyl‐CoA dehydrogenase activity | decrease | 34% inhibition | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of β‐ketothiolase activity | decrease | 25% inhibition | 4 | |
| SYNTHESIS OF KETONE BODY | 2 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of beta oxidation and ketone body formation | decrease | IC50 | 4 | |
| SWELLING | 1 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurements of mitochondrial swelling | Negative | p < 0.05 | 4 | |
| SWELLING | 10 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurements of mitochondrial swelling | Negative | p < 0.05 | 4 | |
| SWELLING | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurements of mitochondrial swelling | increase | p < 0.05 | 4 | |
| OXIDATIVE STRESS | 0.1 nmol/L | 1 hour | human | HepG2 | Measurement of ROS | increase | started to be detectable | 4 |
| OXIDATIVE STRESS | 0.1 μmol/L | 1 hour | human | HepG2 | Measurement of ROS | increase | p < 0.05 | 4 |
| OXIDATIVE STRESS | 1 μmol/L | 1 hour | human | HepG2 | Measurement of ROS | increase | p < 0.01 | 4 |
| OXIDATIVE STRESS | 100 μmol/L | 1 hour | human | HepG2 | Measurement of ROS | increase | p < 0.01 | 4 |
| APOPTOSIS | 100 μmol/L | 8 hours | human | HepG2 | Assessment of cytochrome c release | increase | observable | 4 |
| LATE APOPTOSIS | 100 μmol/L | 8 hours | rat; Sprague–Dawley | hepatocytes | Apoptosis measurement | increase | p < 0.01 | 4 |
| LATE APOPTOSIS | 1 μmol/L | 8 hours | rat; Sprague–Dawley | hepatocytes | Apoptosis measurement | Negative | p < 0.05 | 4 |
| LATE APOPTOSIS | 100 μmol/L | 8 hours | rat; Sprague–Dawley | hepatocytes | Apoptosis measurement | increase | p < 0.01 | 4 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| 3-ketoacyl-CoA thiolase, mitochondrial | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of β‐ketothiolase activity | inhibitor | 25% inhibition | 4 | |
| Short/branched chain specific acyl-CoA dehydrogenase, mitochondrial | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of acyl‐CoA dehydrogenase activity | inhibitor | 34% inhibition | 4 | |
| Cytochrome c | 100 μmol/L | 8 hours | human | HepG2 | Assessment of cytochrome c release | release | observable | 4 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
   |
Danger |
Aggregated GHS information provided by 53 companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H301 (98.11%): Toxic if swallowed [Danger Acute toxicity, oral] H361 (16.98%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H411 (20.75%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P264, P270, P273, P281, P301+P310, P308+P313, P321, P330, P391, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intraperitoneal | 239mg/kg (239mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 10, Pg. 232, 1979. | |
| rat | LD50 | subcutaneous | 1230mg/kg (1230mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 10, Pg. 232, 1979. | |
| mouse | LD50 | intravenous | 77mg/kg (77mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 6, Pg. 341, 1972. | |
| mouse | LD50 | oral | 618mg/kg (618mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 10, Pg. 232, 1979. | |
| mouse | LD50 | subcutaneous | 4120mg/kg (4120mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 10, Pg. 232, 1979. | |
| rat | LD50 | oral | 248mg/kg (248mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 10, Pg. 232, 1979. | |
| mouse | LD50 | intraperitoneal | 146mg/kg (146mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 6, Pg. 341, 1972. | |
| (2-Ethyl-3-benzofuranyl)-(3,5-dibrom-4-hydroxyphenyl)keton | (3,5-Dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran-3-yl)methanone | (3,5-Dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran-3-yl)methanone # |
| (3,5-Dibromo-4-hydroxyphenyl)(2-ethyl-3-benzofuranyl)methanone | (3,5-Dibromo-4-hydroxyphenyl)(2-ethylbenzofuran-3-yl)methanone | (3,5-Dibromo-4-hydroxyphenyl)-(2-ethylbenzofuran-3-yl)methanone |
| (3,5-dibromo-4-hydroxy-phenyl)-(2-ethylbenzofuran-3-yl)methanone | (3,5-dibromo-4-hydroxyphenyl)-(2-ethyl-1-benzofuran-3-yl)methanone | (3,5-dibromo-4-hydroxyphenyl)-(2-ethyl-3-benzofuranyl)methanone |
| 1507-97-7 | 2,6-dibromo-4-[(2-ethyl-1-benzofuran-3-yl)carbonyl]phenol | 2-Ethyl-3-(3,5-dibrom-4-hydroxybenzoyl)benzofuran |
| 2-Ethyl-3-(3,5-dibromo-4-hydroxybenzoyl)benzofuran | 3, 5-Dibromo-4-hydroxyphenyl-2-ethyl-3-benzofuranyl ketone | 3,5-Dibromo-4-hydroxyphenyl 2-ethyl-3-benzofuranyl Ketone |
| 3,5-Dibromo-4-hydroxyphenyl-2-ethyl-3-benzofuranyl ketone | 3,5-dibromo-4-hydroxyphenyl 2-ethylbenzo[b]furan-3-yl ketone | 3-(3,5-Dibromo-4-hydroxybenzoyl)-2-ethylbenzofuran |
| 3-[3,5-DIBROMO-4-HYDROXYBENZOYL]-2-ETHYLBENZOFURAN | 3562-84-3 | 4POG0RL69O |
| 5,10,15-Triphenyl-10,15-dihydro-5H -diindolo[3,2-a:3',2'-c]carbazole | 562B843 | 879713-04-9 |
| A822914 | AC-6162 | ACN-049975 |
| AK468672 | AKOS015895856 | AM20040270 |
| AOB1168 | API0001617 | Acifugan |
| Azubromaron | B4099 | BCP07515 |
| BDBM50158460 | BPBio1_000549 | BRD-K11717138-001-03-0 |
| BRD-K11717138-001-16-2 | BRN 0273668 | BSPBio_000499 |
| Benzbromaron | Benzbromarone (JP17/USAN/INN) | Benzbromarone 1.0 mg/ml in Methanol |
| Benzbromarone [USAN:INN:BAN] | Benzbromarone(USAN) | Benzbromarone, 98% |
| Benzbromarone, European Pharmacopoeia (EP) Reference Standard | Benzbromarone, analytical standard | Benzbromarone, certified reference material, TraceCERT(R) |
| Benzbromarone,(S) | Benzbromaronum | Benzbromaronum [INN-Latin] |
| Benzobromarona | Benzobromarona [INN-Spanish] | Benzofuran, 3-(3,5-dibromo-4-hydroxybenzoyl)-2-ethyl- |
| Besuric | C17H12Br2O3 | CAS-3562-84-3 |
| CCG-37198 | CHEBI:3023 | CHEMBL388590 |
| CPD000058310 | CS-4740 | CTK8F7960 |
| D01056 | DB12319 | DSSTox_CID_2652 |
| DSSTox_GSID_22652 | DSSTox_RID_76675 | DTXSID4022652 |
| Desuric | EINECS 222-630-7 | EX-A1131 |
| Exurate | FT-0083530 | FT-0602164 |
| H503 | HMS1569I21 | HMS2052K05 |
| HMS2093J04 | HMS2096I21 | HMS2230D20 |
| HMS3394K05 | HMS3652H15 | HMS3713I21 |
| HMS3744G15 | HY-B1135 | Harolan |
| Hipurik | KETONE, 3,5-DIBROMO-4-HYDROXYPHENYL 2-ETHYL-3-BENZOFURANYL | KS-0000108Y |
| KS-1292 | Ketone,5-dibromo-4-hydroxyphenyl 2-ethyl-3-benzofuranyl | L 2214 |
| L 2214-Labaz | L-2214 | L2214 |
| L2214-Labaz | LS-87131 | MCULE-5907152599 |
| MFCD00078962 | MJ 10061 | MJ-10061 |
| ML054 | MLS000028522 | MLS000737128 |
| MLS001074105 | MLS001424143 | Max-uric |
| Methanone, (3, 5-dibromo-4-hydroxyphenyl)(2-ethyl-3-benzofuranyl)- | Methanone, (3,5-dibromo-4-hydroxyphenyl)(2-ethyl-3-benzofuranyl)- | Methanone,5-dibromo-4-hydroxyphenyl)(2-ethyl-3-benzofuranyl)- |
| Minuric | NC00334 | NCGC00013895 |
| NCGC00013895-01 | NCGC00013895-02 | NCGC00013895-03 |
| NCGC00013895-04 | NCGC00013895-05 | NCGC00013895-06 |
| NCGC00013895-07 | NCGC00013895-08 | NCGC00013895-09 |
| NCGC00022066-04 | NCI60_041884 | NCI85433 |
| NCIStruc1_001498 | NCIStruc2_001681 | NSC 85433 |
| NSC-759281 | NSC-85433 | NSC759281 |
| NSC85433 | Narcaricin | Normurat |
| Opera_ID_1282 | Oprea1_140235 | Pharmakon1600-01505971 |
| Prestwick0_000370 | Prestwick1_000370 | Prestwick2_000370 |
| Prestwick3_000370 | Prestwick_709 | Q410435 |
| SAM001246877 | SBB057006 | SBI-0055383.P002 |
| SC-09288 | SC-27292 | SC-36260 |
| SCHEMBL48993 | SMR000058310 | SPBio_002420 |
| SR-01000003082 | SR-01000003082-5 | SR-01000003082-6 |
| ST50993903 | SW196858-4 | TPDI |
| Tox21_110041 | Tox21_110041_1 | UNII-4POG0RL69O |
| UNM000001228903 | Uricovac | Urinorm |
| Uroleap (TN) | WHQCHUCQKNIQEC-UHFFFAOYSA-N | ZINC608205 |
| [3,5-bis(bromanyl)-4-oxidanyl-phenyl]-(2-ethyl-1-benzofuran-3-yl)methanone | benzbromarone | cid_2333 |
| s4221 |
| DrugBank Name | Benzbromarone |
| DrugBank | DB12319 |
| CAS Number | 117976-90-6, 125814-23-5, 1507-97-7, 3562-84-3, 879713-04-9 |
| PubChem Compound | 2333 |
| KEGG Drug | D01056 |
| ChEBI | 3023 |
| PharmGKB | PA134852920 |
| ChemSpider | 2243 |
| BindingDB | 50158460.0 |
| Wikipedia | Benzbromarone |