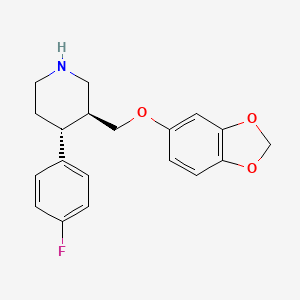
D0441 | paroxetine
N
N06AB05 Paroxetine
[N06AB] Selective serotonin reuptake inhibitors
[N06A] ANTIDEPRESSANTS
[N06] PSYCHOANALEPTICS
[N] Nervous system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex I activity | decrease | p < 0.01 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | decrease | p < 0.001 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | decrease | p < 0.001 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex IV activity | Negative | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex V activity | decrease | p < 0.001 | 3 | |
| ELECTRON TRANSPORT CHAIN | decrease | 35 | ||||||
| ELECTRON TRANSPORT CHAIN | decrease | 35 | ||||||
| ELECTRON TRANSPORT CHAIN | decrease | 35 | ||||||
| ELECTRON TRANSPORT CHAIN | decrease | 35 | ||||||
| GLUCOSE GALACTOSE IC50 RATIO | 37.5 ± 15.9, 36.9 ± 27.9, 1, 37.1 ± 11.3, 32.3 ± 17.8, 1.2 | 4hr | H9c2 cells | high-glucose–galactose cell viability assay with JC-1 mitochondrial membrane potential and ATP-depletion assays (CellTiter-Glo reagent ). | glucose/galactose IC50 ratio (JC-1 IC50 in glucose, JC-1 IC50 in galactose, JC-1 glu/gla, ATP IC50 in glucose, ATP IC50 in galactose, ATP glu/gla ) | 50 | ||
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 50 μM | bovine | heart mitochondria | Measurement of complex I activity | inhibitor | p < 0.01 | 3 | |
| NADH:ubiquinone reductase | inhibitor | 35 | ||||||
| Succinate dehydrogenase | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | inhibitor | p < 0.001 | 3 | |
| Succinate dehydrogenase | inhibitor | 35 | ||||||
| Quinol--cytochrome-c reductase | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | inhibitor | p < 0.001 | 3 | |
| Quinol--cytochrome-c reductase | inhibitor | 35 | ||||||
| Cytochrome c oxidase | 50 μM | bovine | heart mitochondria | Measurement of complex IV activity | Negative | p < 0.05 | 3 | |
| ATP synthase | 50 μM | bovine | heart mitochondria | Measurement of complex V activity | inhibitor | p < 0.001 | 3 | |
| ATP synthase | inhibitor | 35 | ||||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
   |
Danger |
Aggregated GHS information provided by 6 companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 1 of 6 companies. For more detailed information, please visit ECHA C&L website Of the 3 notification(s) provided by 5 of 6 companies with hazard statement code(s): H302 (80%): Harmful if swallowed [Warning Acute toxicity, oral] H360 (60%): May damage fertility or the unborn child [Danger Reproductive toxicity] H361 (20%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H400 (20%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (60%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P264, P270, P273, P281, P301+P312, P308+P313, P330, P391, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
   |
Warning |
Aggregated GHS information provided by 77 companies from 11 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H302 (98.7%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (55.84%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (93.51%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (66.23%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] H361 (12.99%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H411 (40.26%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P261, P264, P270, P271, P273, P280, P281, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P330, P332+P313, P337+P313, P362, P391, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
 |
Warning |
H302: Harmful if swallowed [Warning Acute toxicity, oral] H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] |
P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| (-)-(3S,4R)-4-(p-Fluorophenyl)-3-((3,4-(methylenedioxy)phenoxy)methyl)piperidine | (-)-(3S,4R)-4-(p-Fluorophenyl)-3-((3,4-methylenedioxy)phenoxy)methyl)piperidine | (-)-Paroxetine |
| (-)-trans-4-(4-Fluorophenyl)-3-(3,4-methylenedioxyphenoxymethyl)piperidine | (-)-trans-4-(p-fluorophenyl)-3-[[3,4-(methylenedioxy)phenoxy]methyl]-piperidine | (3S,4R)-3-(1,3-benzodioxol-5-yloxymethyl)-4-(4-fluorophenyl)piperidine |
| (3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine | (3S,4R)-3-[(2H-1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine | (3S,4R)-3-{[(2H-1,3-Benzodioxol-5-yl)oxy]methyl}-4-(4-fluorophenyl)piperidine |
| (3S-trans)-3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)piperidine | (3S-trans)-3-[(1,3-Benzodioxol-5-yl-oxy)methyl]-4-(4-fluorophenyl)piperidine | (3s,4r)-3-((benzo[d][1,3]dioxol-5-yloxy)methyl)-4-(4-fluorophenyl)piperidine |
| 110429-35-1 | 3h-paroxetine | 41VRH5220H |
| 61869-08-7 | 869P087 | 8PR |
| AB00053704-21 | AB00053704_22 | AB00053704_23 |
| AB00514724 | AC-8185 | AHOUBRCZNHFOSL-YOEHRIQHSA-N |
| AKOS015888636 | Aropax | BDBM22416 |
| BDBM50331515 | BIDD:GT0673 | BPBio1_000949 |
| BRD-K37991163-003-02-7 | BRD-K37991163-050-05-1 | BRL 29060 |
| BRL-29060 | BSPBio_000861 | C-19975 |
| C07415 | C19H20FNO3 | CAS-61869-08-7 |
| CC-33477 | CHEBI:7936 | CHEMBL1708 |
| CHEMBL490 | Casbol | D02362 |
| DB00715 | DSSTox_CID_3425 | DSSTox_GSID_23425 |
| DSSTox_RID_77022 | DTXSID3023425 | DivK1c_006884 |
| FG 7051 | FG-7051 | FT-0085087 |
| Frosinor | GTPL4790 | HMS2090H05 |
| HSDB 7175 | KBio1_001828 | KBio2_002232 |
| KBio2_004800 | KBio2_007368 | KBioSS_002232 |
| KS-00000JK2 | LS-114249 | Motivan |
| NCGC00025355-02 | NCGC00025355-03 | NCGC00025355-04 |
| NCGC00025355-05 | NCGC00025355-06 | NCGC00025355-07 |
| NCGC00025355-08 | NCGC00025355-09 | NCGC00025355-12 |
| NCGC00182968-01 | NNC-20-7051 | PAROXETINE HCL HEMIHYDRATE |
| Paroxetin HCL | Paroxetina | Paroxetina [INN-Spanish] |
| Paroxetine (TN) | Paroxetine (USP/INN) | Paroxetine Hydrochloride Anhydrous EP Impurity E |
| Paroxetine USP Related Compound D;[(3S,4R)-4-(p-Fluorophenyl)-3-piperidyl](1,3-dioxa-5-indanyloxy)methane; | Paroxetine [USAN:INN:BAN] | Paroxetine.HCl |
| Paroxetinum | Paroxetinum [INN-Latin] | PaxPar |
| Paxetil | Paxil | Paxil CR |
| Pexeva | Piperidine, 3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)-, (3S,4R)- | Piperidine, 3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)-, (3S-trans)- |
| Piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-, (3S-trans)- | Piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-,(3S,4R)- | Prestwick3_000851 |
| Q408471 | SBI-0051908.P002 | SC-16100 |
| SC-22977 | SCHEMBL27799 | Seroxat |
| SpecPlus_000788 | Spectrum5_001665 | Spectrum_001752 |
| Tox21_113123 | Tox21_113123_1 | UNII-41VRH5220H |
| ZINC527386 | [3H]Paroxetine | cis-Paroxetine |
| paroxetine | paroxetine hydrochloride (anhydrous or hemihydrate) | piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-, (3S,4R)- |
| DrugBank Name | paroxetine |
| DrugBank | DB00715 |
| CAS Number | 110429-35-1, 61869-08-7, 78246-49-8 |
| PubChem Compound | 43815 |
| KEGG Compound ID | C07415 |
| KEGG Drug | D02362 |
| ChEBI | 7936 |
| PharmGKB | PA450801 |
| ChemSpider | 39888 |
| BindingDB | 50331515.0 |
| TTD | DAP001428 |
| Wikipedia | Paroxetine |