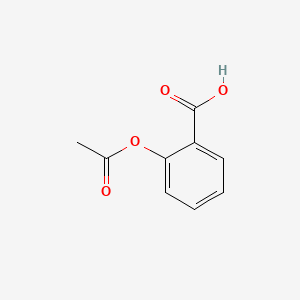
D0010 | Acetylsalicylic acid
M
A
B
C
N
N02BA71 Acetylsalicylic acid, combinations with psycholeptics
[N02BA] Salicylic acid and derivatives
[N02B] OTHER ANALGESICS AND ANTIPYRETICS
[N02] ANALGESICS
[N] Nervous system
N02BA51 Acetylsalicylic acid, combinations excl. psycholeptics
[N02BA] Salicylic acid and derivatives
[N02B] OTHER ANALGESICS AND ANTIPYRETICS
[N02] ANALGESICS
[N] Nervous system
N02BA01 Acetylsalicylic acid
[N02BA] Salicylic acid and derivatives
[N02B] OTHER ANALGESICS AND ANTIPYRETICS
[N02] ANALGESICS
[N] Nervous system
N02AJ18 Oxycodone and acetylsalicylic acid
[N02AJ] Opioids in combination with non-opioid analgesics
[N02A] OPIOIDS
[N02] ANALGESICS
[N] Nervous system
N02AJ07 Codeine and acetylsalicylic acid
[N02AJ] Opioids in combination with non-opioid analgesics
[N02A] OPIOIDS
[N02] ANALGESICS
[N] Nervous system
N02AJ02 Dihydrocodeine and acetylsalicylic acid
[N02AJ] Opioids in combination with non-opioid analgesics
[N02A] OPIOIDS
[N02] ANALGESICS
[N] Nervous system
M01BA03 Acetylsalicylic acid and corticosteroids
[M01BA] Antiinflammatory/antirheumatic agents in combination with corticosteroids
[M01B] ANTIINFLAMMATORY/ANTIRHEUMATIC AGENTS IN COMBINATION
[M01] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS
[M] Musculoskeletal system
C10BX12 Atorvastatin, acetylsalicylic acid and perindopril
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BX08 Atorvastatin and acetylsalicylic acid
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BX06 Atorvastatin, acetylsalicylic acid and ramipril
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BX05 Rosuvastatin and acetylsalicylic acid
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BX04 Simvastatin, acetylsalicylic acid and ramipril
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BX02 Pravastatin and acetylsalicylic acid
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BX01 Simvastatin and acetylsalicylic acid
[C10BX] HMG CoA reductase inhibitors, other combinations
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C07FX04 Bisoprolol and acetylsalicylic acid
[C07FX] Beta blocking agents, other combinations
[C07F] BETA BLOCKING AGENTS, OTHER COMBINATIONS
[C07] BETA BLOCKING AGENTS
[C] Cardiovascular system
C07FX03 Metoprolol and acetylsalicylic acid
[C07FX] Beta blocking agents, other combinations
[C07F] BETA BLOCKING AGENTS, OTHER COMBINATIONS
[C07] BETA BLOCKING AGENTS
[C] Cardiovascular system
C07FX02 Sotalol and acetylsalicylic acid
[C07FX] Beta blocking agents, other combinations
[C07F] BETA BLOCKING AGENTS, OTHER COMBINATIONS
[C07] BETA BLOCKING AGENTS
[C] Cardiovascular system
B01AC56 Acetylsalicylic acid, combinations with proton pump inhibitors
[B01AC] Platelet aggregation inhibitors excl. heparin
[B01A] ANTITHROMBOTIC AGENTS
[B01] ANTITHROMBOTIC AGENTS
[B] Blood and blood forming organs
B01AC06 Acetylsalicylic acid
[B01AC] Platelet aggregation inhibitors excl. heparin
[B01A] ANTITHROMBOTIC AGENTS
[B01] ANTITHROMBOTIC AGENTS
[B] Blood and blood forming organs
A01AD05 Acetylsalicylic acid
[A01AD] Other agents for local oral treatment
[A01A] STOMATOLOGICAL PREPARATIONS
[A01] STOMATOLOGICAL PREPARATIONS
[A] Alimentary tract and metabolism
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| TRANSMEMBRANE POTENTIAL | NR | 24hr | rat | hepatocytes | tetramethylrhodamine ethyl ester (TMRE) | Negative | AC50 (μM) | 40 |
| ELECTROPHORETIC UNCOUPLING | 278 | |||||||
| MEMBRANE POTENTIAL | 335.5 µM | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | decrease | EC20 | 36 |
| RESPIRATION | > 800 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | decrease | EC20 | 36 |
| RESPIRATION | 149.8 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | decrease | EC20 | 36 |
| STATE 2 RESPIRATION | 100 nmol/mg mitochondrial protein | rat | isolated rat liver mitochondria | State 2 respiration ( 96-well plate format using a phosphorescent oxygen-sensitive probe MitoXpress) | Negative | UC50 (nmol/mg mitochondrial protein) | 40 | |
| STATE 3 RESPIRATION | 100 nmol/mg mitochondrial protein | rat | isolated rat liver mitochondria | State 3 respiration ( 96-well plate format using a phosphorescent oxygen-sensitive probe MitoXpress) | Negative | IC50 (nmol/mg mitochondrial protein) | 40 | |
| OXYGEN CONSUMPTION RATE (OCR) | 1 mM | 2 minutes | human | HepG2 | Measurement of OCR | Negative | EC50 | 7 |
| ELECTRON TRANSPORT CHAIN | decrease | 35 | ||||||
| ELECTRON TRANSPORT CHAIN | decrease | 35 | ||||||
| ECAR | 1 mM | 2 minutes | human | HepG2 | Measurement of ECAR | Negative | EC50 | 7 |
| LIPID METABOLISM | NR | 24hr | rat | hepatocytes | LipidTox, for neutral lipid accumulation, to evaluate lipid content. | Negative | AC50 (μM) | 40 |
| GLUTATHIONE METABOLISM | NR | 24hr | rat | hepatocytes | glutathion depletion: cells were incubated with 50 μM monochlorobimane with 6 μg/ml Hoechst 33342 | Negative | AC50 (μM) | 40 |
| SWELLING | > 800 µM | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | increase | EC20 | 36 |
| OXIDATIVE STRESS | 5 μmol/ml | 24 hours | human | HepG2 | ROS measurement | Negative | p < 0.05 | 2 |
| OXIDATIVE STRESS | 5 μmol/ml | 48 hours | human | HepG2 | ROS measurement | Negative | p < 0.05 | 2 |
| OXIDATIVE STRESS | 10 μmol/ml | 24 hours | human | HepG2 | ROS measurement | increase | p < 0.05 | 2 |
| OXIDATIVE STRESS | 10 μmol/ml | 48 hours | human | HepG2 | ROS measurement | increase | p < 0.05 | 2 |
| ROS PRODUCTION | NR | rat | hepatocytes | use CM-H2DCFDA to monitor reactive oxygen species | Negative | AC50 (μM) | 40 | |
| CYTOCHROME C RELEASE | NR | 24hr | rat | hepatocytes | cytochrome c release (anti-cytochrome c antibody ) | Negative | AC50 (μM) | 40 |
| ER STRESS-INDUCED | NR | 24hr | rat | hepatocytes | DNA damage 153 induction (GADD153 antibodies) for ER-stress induced apoptosis | Negative | AC50 (μM) | 40 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | inhibitor | 35 | ||||||
| NADH:ubiquinone reductase | > 800 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | inhibit | EC20 | 36 |
| Succinate dehydrogenase | 149.8 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | inhibit | EC20 | 36 |
| Cytochrome c oxidase | inhibitor | 35 | ||||||
| Reactive oxygen species | 5 μmol/ml | 24 hours | human | HepG2 | ROS measurement | Negative | p < 0.05 | 2 |
| Reactive oxygen species | 5 μmol/ml | 48 hours | human | HepG2 | ROS measurement | Negative | p < 0.05 | 2 |
| Reactive oxygen species | 10 μmol/ml | 24 hours | human | HepG2 | ROS measurement | increase | p < 0.05 | 2 |
| Reactive oxygen species | 10 μmol/ml | 48 hours | human | HepG2 | ROS measurement | increase | p < 0.05 | 2 |
| Cytochrome c | > 200 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 181 companies from 19 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 1 of 181 companies. For more detailed information, please visit ECHA C&L website Of the 18 notification(s) provided by 180 of 181 companies with hazard statement code(s): H302 (92.22%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (35%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (37.78%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (33.89%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
  |
Danger |
H302: Harmful if swallowed [Warning Acute toxicity, oral] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H334: May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory] H360: May damage fertility or the unborn child [Danger Reproductive toxicity] H370: Causes damage to organs [Danger Specific target organ toxicity, single exposure] H372: Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure] |
P201, P202, P260, P261, P264, P270, P280, P281, P285, P301+P312, P304+P341, P305+P351+P338, P307+P311, P308+P313, P314, P321, P330, P337+P313, P342+P311, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
  |
Danger |
H302: Harmful if swallowed [Warning Acute toxicity, oral] H316: Causes mild skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H334: May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory] H360: May damage fertility or the unborn child [Danger Reproductive toxicity] H371: May cause damage to organs [Warning Specific target organ toxicity, single exposure] H373: Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] |
P201, P202, P260, P261, P264, P270, P280, P281, P285, P301+P312, P304+P341, P305+P351+P338, P308+P313, P309+P311, P314, P330, P332+P313, P337+P313, P342+P311, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| women | TDLo | oral | 800mg/kg (800mg/kg) | American Journal of Emergency Medicine. Vol. 7, Pg. 409, 1989. | |
| man | TDLo | oral | 857mg/kg (857mg/kg) | Human Toxicology. Vol. 7, Pg. 161, 1988. | |
| human | TDLo | oral | 2880mg/kg/8W (2880mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 33, Pg. 631, 1983. | |
| infant | TDLo | oral | 120mg/kg (120mg/kg) | British Medical Journal. Vol. 1, Pg. 1081, 1979. | |
| child | TDLo | oral | 39mg/kg/13D-I (39mg/kg) | liver: "hepatitis (hepatocellular necrosis), diffuse" | American Journal of Diseases of Children. Vol. 139, Pg. 453, 1985. |
| dog | LD50 | intravenous | 681mg/kg (681mg/kg) | behavioral: analgesia | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 149, Pg. 571, 1964. |
| dog | LD50 | oral | 700mg/kg (700mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 21, Pg. 719, 1971. | |
| guinea pig | LD50 | oral | 1075mg/kg (1075mg/kg) | Journal of the American Pharmaceutical Association, Scientific Edition. Vol. 47, Pg. 479, 1958. | |
| man | LDLo | unreported | 294mg/kg (294mg/kg) | "Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. | |
| mouse | LD50 | unreported | 1350mg/kg (1350mg/kg) | United States Patent Document. Vol. #5240918, | |
| rat | LD50 | intraperitoneal | 340mg/kg (340mg/kg) | Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 62, Pg. 11, 1966. | |
| women | TDLo | oral | 525mg/kg/5D-I (525mg/kg) | liver: "hepatitis (hepatocellular necrosis), diffuse" | Annals of Internal Medicine. Vol. 80, Pg. 74, 1974. |
| women | TDLo | rectal | 4550mg/kg (4550mg/kg) | Annals of Pharmacotherpy. Vol. 28, Pg. 467, 1994. | |
| human | TDLo | oral | 480mg/kg/7D-I (480mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 25, Pg. 281, 1975. | |
| man | TDLo | oral | 1625mg/kg (1625mg/kg) | behavioral: coma | Clinical Pediatrics Vol. 24, Pg. 678, 1985. |
| rabbit | LD50 | oral | 1010mg/kg (1010mg/kg) | behavioral: changes in motor activity (specific assay) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 24(3), Pg. 43, 1980. |
| hamster | LD50 | oral | 3500mg/kg (3500mg/kg) | Archives of Toxicology, Supplement. Vol. 7, Pg. 365, 1984. | |
| mouse | LD50 | subcutaneous | 1020mg/kg (1020mg/kg) | Chemical and Pharmaceutical Bulletin. Vol. 28, Pg. 1237, 1980. | |
| rat | LD50 | rectal | 790mg/kg (790mg/kg) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 67, 1969. | |
| women | TDLo | oral | 480mg/kg/5D-I (480mg/kg) | kidney, ureter, and bladder: "changes in tubules (including acute renal failure, acute tubular necrosis)" | New England Journal of Medicine. Vol. 296, Pg. 418, 1977. |
| human | TDLo | oral | 669mg/kg/11D (669mg/kg) | liver: liver function tests impaired | American Journal of Hospital Pharmacy. Vol. 35, Pg. 330, 1978. |
| human | TDLo | oral | 1050mg/kg/14D (1050mg/kg) | vascular: other changes | Clinical Pharmacology and Therapeutics Vol. 67, Pg. 530, 2000. |
| mouse | LD50 | intraperitoneal | 167mg/kg (167mg/kg) | United States Patent Document. Vol. #4376771, | |
| mouse | LD50 | oral | 250mg/kg (250mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 5, Pg. 572, 1955. | |
| mammal (species unspecified) | LD50 | oral | 1750mg/kg (1750mg/kg) | Indian Journal of Medical Research. Vol. 81, Pg. 621, 1985. | |
| man | TDLo | oral | 13036mg/kg/5Y (13036mg/kg) | Annals of Internal Medicine. Vol. 126, Pg. 665, 1997. | |
| child | TDLo | oral | 10mg/kg/1D-I (10mg/kg) | Clinical Toxicology. Vol. 18, Pg. 247, 1981. | |
| rat | LD50 | oral | 200mg/kg (200mg/kg) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 67, 1969. | |
| child | LDLo | oral | 104mg/kg (104mg/kg) | Lancet. Vol. 2, Pg. 809, 1952. | |
| (non-d)Acetylsalicylic Acid-d3 | 11126-35-5 | 156865-15-5 |
| 170197-EP2275413A1 | 170197-EP2287156A1 | 1777-EP2269989A1 |
| 1777-EP2269990A1 | 1777-EP2272825A2 | 1777-EP2275420A1 |
| 1777-EP2277865A1 | 1777-EP2280008A2 | 1777-EP2281563A1 |
| 1777-EP2281815A1 | 1777-EP2281818A1 | 1777-EP2292227A2 |
| 1777-EP2295055A2 | 1777-EP2298764A1 | 1777-EP2298765A1 |
| 1777-EP2298768A1 | 1777-EP2298776A1 | 1777-EP2305219A1 |
| 1777-EP2305260A1 | 1777-EP2305640A2 | 1777-EP2305652A2 |
| 1777-EP2308510A1 | 1777-EP2311453A1 | 1777-EP2314590A1 |
| 1777-EP2314593A1 | 1777-EP2316459A1 | 1777-EP2371811A2 |
| 186947-EP2270113A1 | 186947-EP2272935A1 | 1oxr |
| 2-(Acetyloxy)-benzoic acid | 2-(Acetyloxy)benzoic acid | 2-(acetyloxy)benzoate |
| 2-Acetoxybenzenecarboxylic acid | 2-Acetoxybenzoate | 2-Acetoxybenzoic acid |
| 2-Acetoxybenzoic acid | 2-Acetylsalicyclic acid | 2-Carboxyphenyl acetate |
| 2-acetoxy benzoic acid | 2-acetyloxybenzoic acid | 24189-EP2295409A1 |
| 24189-EP2314590A1 | 4-10-00-00138 (Beilstein Handbook Reference) | 50-78-2 |
| 6474-EP1441224A2 | 6474-EP2272832A1 | 6474-EP2275420A1 |
| 6474-EP2277861A1 | 6474-EP2277875A2 | 6474-EP2298757A2 |
| 6474-EP2298764A1 | 6474-EP2298765A1 | 6474-EP2314585A1 |
| 8-hour Bayer | 99512-66-0 | A 5376 |
| A.S.A | A.S.A. | A.S.A. empirin |
| AB00051918-08 | AB00051918_09 | AB00051918_10 |
| AB1003266 | AC 5230 | ACETYLSALICYLIC ACID |
| ACMC-209kpz | AI3-02956 | AIN |
| AKOS000118884 | ANW-31125 | ASA |
| ASA | Acenterine | Acesal |
| Acesan | Acetard | Aceticyl |
| Acetilsalicilico | Acetilum acidulatum | Acetisal |
| Acetonyl | Acetophen | Acetosal |
| Acetosalic acid | Acetosalin | Acetoxybenzoic acid |
| Acetylin | Acetylsal | Acetylsalicyclic acid |
| Acetylsalicylate | Acetylsalicylic Acid 1.0 mg/ml in Acetonitrile | Acetylsalicylic acid for peak identification, European Pharmacopoeia (EP) Reference Standard |
| Acetylsalicylic acid, 99% | Acetylsalicylic acid, >=99% | Acetylsalicylic acid, >=99.0% |
| Acetylsalicylic acid, BioReagent, plant cell culture tested, >=99.0% | Acetylsalicylic acid, European Pharmacopoeia (EP) Reference Standard | Acetylsalicylic acid, Vetec(TM) reagent grade, >=99% |
| Acetylsalicylic acid, analytical standard | Acetylsalicylic acid-carboxy-14c | AcetylsalicylicAcid |
| Acetylsalicylsaeure | Acetylsalicylsaure | Acetylsalicylsaure [German] |
| Acetylsalycilic acid | Acetyonyl | Acetysal |
| Acetysalicylic acid | Acide acetylsalicylique | Acide acetylsalicylique [French] |
| Acido O-acetil-benzoico | Acido O-acetil-benzoico [Italian] | Acido acetilsalicilico |
| Acido acetilsalicilico [Italian] | Acidum acetylsalicylicum | Acimetten |
| Acisal | Acylpyrin | Adiro |
| Aloxiprimum | Arthritis Pain Formula Maximum Strength (Salt/Mix) | Asacard |
| Asagran | Asaphen | Asatard |
| Ascoden-30 | Ascolong | Ascriptin (Salt/Mix) |
| Aspalon | Aspalon (JAN) | Aspec |
| Aspergum | Aspir-Mox | Aspirdrops |
| Aspirin | Aspirin | Aspirin (Acetyl Salicylic Acid), Pharmaceutical Secondary Standard |
| Aspirin (JP17/USP) | Aspirin USP (2080B) | Aspirin USP (3080) |
| Aspirin USP-26 | Aspirin [BAN:JAN] | Aspirin [USP:BAN:JAN] |
| Aspirin form II | Aspirin, British Pharmacopoeia (BP) Reference Standard | Aspirin, United States Pharmacopeia (USP) Reference Standard |
| Aspirin, meets USP testing specifications | Aspirin,(S) | AspirinTest2 |
| Aspirina 03 | Aspirine | Asprin |
| Aspro | Aspro Clear | Aspropharm |
| Asteric | Azetylsalizylsaeure | Azetylsalizylsaure |
| BAY-1019036 | BBL005469 | BCP21790 |
| BDBM22360 | BIDD:GT0118 | BRN 0779271 |
| BSYNRYMUTXBXSQ-UHFFFAOYSA-N | Bay E4465 | Bay-e-4465 |
| Bayer | Bayer Aspirin 8 Hour | Bayer Buffered |
| Bayer Children's Aspirin | Bayer Enteric 325 mg Regular Strength | Bayer Enteric 500 mg Arthritis Strength |
| Bayer Enteric 81 mg Adult Low Strength | Bayer Extra Strength Aspirin for Migraine Pain | Bayer Plus |
| Benaspir | Benzoic acid, 2-(acetyloxy)- | Benzoicacid, 2-(acetyloxy)- |
| Bi-prin | Bialpirina | Bialpirinia |
| C01405 | CAS-50-78-2 | CCG-39490 |
| CCRIS 3243 | CHEBI:15365 | CHEMBL25 |
| CS-2001 | CTK1G9661 | Cardioaspirin |
| Cardioaspirina | Cemirit | Certified Reference Material |
| Claradin | Clariprin | Colfarit |
| Colsprin | Contrheuma retard | D00109 |
| D41527A7-A9EB-472D-A7FC-312821130549 | DB00945 | DS-017139 |
| DSSTox_CID_108 | DSSTox_GSID_20108 | DSSTox_RID_75372 |
| DTXSID5020108 | Decaten | Delgesic |
| DivK1c_000555 | Dolean pH 8 | Duramax |
| Durlaza | Durlaza ER | EC 200-064-1 |
| ECM | EINECS 200-064-1 | EU-0100038 |
| Easprin | Easprin (TN) | Ecolen |
| Ecotrin | Empirin | Empirin with Codeine |
| Empirin with Codeine (Salt/Mix) | Endosprin | Endydol |
| Entericin | Enterophen | Enterosarein |
| Enterosarine | Entrophen | Epitope ID:114151 |
| Extren | F2191-0068 | FT-0655181 |
| GTPL4139 | Globentyl | Globoid |
| H740 | HMS1920E13 | HMS2090G03 |
| HMS2091K13 | HMS2233L18 | HMS3260G17 |
| HMS3372N15 | HMS3656N14 | HMS3715P19 |
| HMS501L17 | HSDB 652 | HY-14654 |
| Helicon | IDI1_000555 | Idragin |
| InChI=1/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12 | Istopirin | KBio1_000555 |
| KBio2_001725 | KBio2_002271 | KBio2_004293 |
| KBio2_004839 | KBio2_006861 | KBio2_007407 |
| KBio3_002149 | KBio3_002751 | KBioGR_000398 |
| KBioGR_002271 | KBioSS_001725 | KBioSS_002272 |
| KS-00000ULP | KSC269M6D | Kapsazal |
| Kyselina 2-acetoxybenzoova | Kyselina 2-acetoxybenzoova [Czech] | Kyselina acetylsalicylova |
| Kyselina acetylsalicylova [Czech] | LP00038 | LS-143 |
| Levius | Lopac-A-5376 | Lopac0_000038 |
| MCULE-3199019536 | METHANONE,(2-CHLORO-3-PYRIDINYL)CYCLOBUTYL- | MFCD00002430 |
| MLS001055329 | MLS001066332 | MLS001336045 |
| MLS001336046 | Magnecyl | Measurin |
| Medisyl | Micrainin (Salt/Mix) | Micristin |
| Miniasal | NCGC00015067-01 | NCGC00015067-02 |
| NCGC00015067-03 | NCGC00015067-04 | NCGC00015067-05 |
| NCGC00015067-06 | NCGC00015067-07 | NCGC00015067-08 |
| NCGC00015067-09 | NCGC00015067-10 | NCGC00015067-11 |
| NCGC00015067-12 | NCGC00015067-13 | NCGC00015067-14 |
| NCGC00090977-01 | NCGC00090977-02 | NCGC00090977-03 |
| NCGC00090977-04 | NCGC00090977-05 | NCGC00090977-06 |
| NCGC00090977-07 | NCGC00254034-01 | NCGC00259666-01 |
| NCGC00260723-01 | NCI60_002222 | NINDS_000555 |
| NSC 27223 | NSC-27223 | NSC-406186 |
| NSC-755899 | NSC27223 | NSC406186 |
| NSC755899 | Neuronika | Novid |
| Nu-seals | Nu-seals aspirin | O-Acetylsalicylic acid |
| O-Acetylsalicylic acid | O-Acetylsalicylic acid | PL-2200 |
| Percodan (Salt/Mix) | Percodan Demi (Salt/Mix) | Persistin |
| Pharmacin | Pharmakon1600-01500130 | Pirseal |
| Polopirin | Polopiryna | Pravigard PAC (Salt/Mix) |
| Premaspin | PubChem20190 | Q18216 |
| R16CO5Y76E | RTX-012224 | Rheumin tabletten |
| Rheumintabletten | Rhodine | Rhonal |
| Ronal | S-211 | SBB015069 |
| SBI-0050027.P004 | SC-18564 | SC-61857 |
| SCHEMBL1353 | SMR000059138 | SP 189 |
| SPBio_001838 | SPECTRUM1500130 | SR-01000075668 |
| SR-01000075668-1 | SR-01000075668-4 | SR-01000075668-6 |
| ST075414 | STL137674 | STR01551 |
| SW199665-2 | Salacetin | Salcetogen |
| Saletin | Salicylic acid acetate | Salicylic acid, acetate |
| Salicylic acid, acetyl- | Salospir | Salycylacetylsalicylic acid |
| Solfrin | Solprin | Solprin acid |
| Solpyron | Solupsan | Soma Compound (Salt/Mix) |
| Spectrum2_001899 | Spectrum3_001295 | Spectrum4_000099 |
| Spectrum5_000740 | Spectrum_001245 | Spira-Dine |
| St. Joseph | St. Joseph Aspirin for Adults | Tasprin |
| Temperal | Toldex | Tox21_110076 |
| Tox21_110076_1 | Tox21_202117 | Tox21_300146 |
| Tox21_500038 | Triaminicin | Triple-sal |
| UNII-R16CO5Y76E | UNM-0000306102 | WLN: QVR BOV1 |
| XAXA | Yasta | Z234893989 |
| ZINC53 | ZORprin | acetyl salicyclic acid |
| acetyl salicylic acid | acetyl-salicylic acid | acide 2-(acetyloxy)benzoique |
| aspirin | aspirin (acetylsalicylic acid) | aspirin-acetylsalicylic-acid |
| cMAP_000006 | cid_2244 | component of Ascodeen-30 |
| component of Ascodeen-30 (Salt/Mix) | component of Coricidin | component of Darvon with A.S.A |
| component of Darvon with A.S.A (Salt/Mix) | component of Midol | component of Persistin |
| component of Robaxisal | component of St. Joseph Cold Tablets | component of Synirin |
| component of Zactirin | component of Zactirin (Salt/Mix) | o-(Acetyloxy)benzoate |
| o-(Acetyloxy)benzoic acid | o-Acetoxybenzoate | o-Acetoxybenzoic acid |
| o-Carboxyphenyl acetate | o-acetyl-salicylic acid | s3017 |
| DrugBank Name | Acetylsalicylic acid |
| DrugBank | DB00945 |
| CAS Number | 11126-35-5, 156865-15-5, 50-78-2, 52080-78-1, 921943-73-9, 98201-60-6, 99512-66-0 |
| PubChem Compound | 2244 |
| KEGG Compound ID | C01405 |
| KEGG Drug | D00109 |
| PubChem.Substance | 46505803 |
| ChEBI | 15365 |
| PharmGKB | PA448497 |
| ChemSpider | 2157 |
| BindingDB | 22360.0 |
| TTD | DAP000843 |
| Wikipedia | Aspirin |
| HET | AIN |
| DPD | 150 |